20:
23:
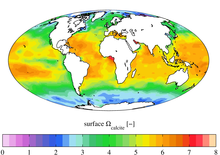
The graphic presents the present-day annual mean surface omega calcite: the normalised saturation state of calcite. Areas with a value less an 1 indicate a likeliness for dissolution (undersaturated) while a value over 1 indicates areas less likely for dissolution
117:
saturation state of seawater. The calcite saturation horizon is where Ω =1; dissolution proceeds slowly below this depth. The lysocline is the depth that this dissolution impacts is again notable, also known as the inflection point with sedimentary
59:, content values reaching 85–95%. This area is then spanned hundreds of meters by the transition zone, ending in the abyssal depths with 0% concentration. The lysocline is the upper bound of the transition zone, where amounts of CaCO
55:
content in sediment varies with different depths of the ocean, spanned by levels of separation known as the transition zone. In the mid-depth area of the ocean, sediments are rich in CaCO
48:
increases dramatically because of a pressure effect. While the lysocline is the upper bound of this transition zone of calcite saturation, the CCD is the lower bound of this zone.
157:
The depth of the CCD varies as a function of the chemical composition of the seawater and its temperature. Specifically, it is the deep waters that are undersaturated with
138:
content are values 2–10%. Hence, the lysocline and CCD are not equivalent. The lysocline and compensation depth occur at greater depths in the
416:
Volat, J. L.; Pastouret, L.; V. G., Colette (1980). "Dissolution and carbonate fluctuations in
Pleistocene deep-sea cores: A review".
134:(CCD) occurs at the depth that the rate of calcite to the sediments is balanced with the dissolution flux, the depth at which the CaCO
94:, calcite solubility increases, causing supersaturated water above the saturation depth, allowing for preservation and burial of CaCO
255:
Shiraiwa, Y. (2003). "Physiological regulation of carbon fixation in the photosynthesis and calcification of coccolithophorids".
234:
183:
131:
37:
365:
Zeebe, R. E. (2012). "History of
Seawater Carbonate Chemistry, Atmospheric CO2, and Ocean Acidification".
98:
on the seafloor. However, this creates undersaturated seawater below the saturation depth, preventing CaCO
301:
Sigman, D. M.; Boyle, E. A. (2000). "Glacial/interglacial variations in atmospheric carbon dioxide".
214:
19:
169:
continues to increase, the CCD can be expected to decrease in depth, as the ocean's acidity rises.
90:) die, they tend to fall downwards without dissolving. As depth and pressure increases within the
151:
529:
524:
468:
425:
374:
310:
8:
480:
188:
472:
429:
386:
378:
314:
16:
Depth in the ocean below which the rate of dissolution of calcite increases dramatically
500:
398:
342:
268:
492:
484:
441:
437:
390:
334:
326:
280:
272:
230:
226:
158:
504:
402:
476:
433:
382:
346:
318:
264:
222:
257:
Comparative
Biochemistry and Physiology Part B: Biochemistry and Molecular Biology
178:
161:
primarily because its solubility increases strongly with increasing pressure and
75:
459:
Broecker, W. S. (2009). "Wally's Quest to
Understand the Ocean's CaCO3 Cycle".
166:
139:
63:
content begins to noticeably drop from the mid-depth 85–95% sediment. The CaCO
518:
488:
445:
394:
330:
276:
143:
68:
496:
338:
284:
91:
83:
213:
Broecker, W. S. (2003), Holland, Heinrich D.; Turekian, Karl K. (eds.),
322:
87:
41:
162:
147:
45:
165:
and decreasing temperature. As the atmospheric concentration of
103:
71:
at the lower bound, known as the calcite compensation depth.
33:
86:(which often have shells made of calcite or its polymorph,
40:(CCD), usually around 5 km, below which the rate of
415:
516:
367:Annual Review of Earth and Planetary Sciences
125:
300:
458:
254:
212:
146:(4000–5000 m), and at greater depths in
18:
517:
364:
208:
206:
204:
481:10.1146/annurev.marine.010908.163936
360:
358:
356:
296:
294:
74:Shallow marine waters are generally
387:10.1146/annurev-earth-042711-105521
13:
201:
14:
541:
353:
291:
106:as the shells start to dissolve.
215:"6.19 – The Oceanic CaCO3 Cycle"
461:Annual Review of Marine Science
452:
409:
248:
227:10.1016/b0-08-043751-6/06119-3
221:, Pergamon, pp. 529–549,
1:
269:10.1016/S1096-4959(03)00221-5
194:
122:versus various water depths.
438:10.1016/0025-3227(80)90138-3
184:Carbonate compensation depth
38:carbonate compensation depth
7:
172:
10:
546:
142:(5000–6000 m) than in the
132:calcite compensation depth
126:Calcite compensation depth
219:Treatise on Geochemistry
109:The equation Ω = X /K'
25:
22:
67:content drops to 0%
32:is the depth in the
473:2009ARMS....1....1B
430:1980MGeol..34....1V
379:2012AREPS..40..141Z
315:2000Natur.407..859S
189:Ocean acidification
36:dependent upon the
148:equatorial regions
113:expresses the CaCO
26:
309:(6806): 859–869.
159:calcium carbonate
24:(oversaturation).
537:
509:
508:
456:
450:
449:
413:
407:
406:
362:
351:
350:
323:10.1038/35038000
298:
289:
288:
252:
246:
245:
244:
243:
210:
84:marine organisms
78:in calcite, CaCO
545:
544:
540:
539:
538:
536:
535:
534:
515:
514:
513:
512:
457:
453:
414:
410:
363:
354:
299:
292:
253:
249:
241:
239:
237:
211:
202:
197:
179:Biological pump
175:
137:
128:
121:
116:
112:
101:
97:
81:
66:
62:
58:
54:
17:
12:
11:
5:
543:
533:
532:
527:
511:
510:
451:
418:Marine Geology
408:
373:(1): 141–165.
352:
290:
263:(4): 775–783.
247:
235:
199:
198:
196:
193:
192:
191:
186:
181:
174:
171:
167:carbon dioxide
135:
127:
124:
119:
114:
110:
102:burial on the
99:
95:
79:
76:supersaturated
64:
60:
56:
52:
15:
9:
6:
4:
3:
2:
542:
531:
528:
526:
523:
522:
520:
506:
502:
498:
494:
490:
486:
482:
478:
474:
470:
466:
462:
455:
447:
443:
439:
435:
431:
427:
423:
419:
412:
404:
400:
396:
392:
388:
384:
380:
376:
372:
368:
361:
359:
357:
348:
344:
340:
336:
332:
328:
324:
320:
316:
312:
308:
304:
297:
295:
286:
282:
278:
274:
270:
266:
262:
258:
251:
238:
236:9780080437514
232:
228:
224:
220:
216:
209:
207:
205:
200:
190:
187:
185:
182:
180:
177:
176:
170:
168:
164:
160:
155:
153:
152:polar regions
149:
145:
141:
133:
123:
107:
105:
93:
89:
85:
82:, because as
77:
72:
70:
69:concentration
49:
47:
43:
39:
35:
31:
21:
530:Oceanography
525:Geochemistry
464:
460:
454:
421:
417:
411:
370:
366:
306:
302:
260:
256:
250:
240:, retrieved
218:
156:
129:
108:
92:water column
73:
50:
29:
27:
467:(1): 1–18.
424:(1): 1–28.
42:dissolution
519:Categories
242:2019-10-17
195:References
489:1941-1405
446:0025-3227
395:0084-6597
331:1476-4687
277:1096-4959
104:sea floor
88:aragonite
30:lysocline
505:45348785
497:21141027
403:18682623
339:11057657
285:14662302
173:See also
163:salinity
150:than in
140:Atlantic
469:Bibcode
426:Bibcode
375:Bibcode
347:7136822
311:Bibcode
144:Pacific
46:calcite
503:
495:
487:
444:
401:
393:
345:
337:
329:
303:Nature
283:
275:
233:
501:S2CID
399:S2CID
343:S2CID
34:ocean
493:PMID
485:ISSN
442:ISSN
391:ISSN
335:PMID
327:ISSN
281:PMID
273:ISSN
231:ISBN
130:The
118:CaCO
51:CaCO
28:The
477:doi
434:doi
383:doi
319:doi
307:407
265:doi
261:136
223:doi
44:of
521::
499:.
491:.
483:.
475:.
463:.
440:.
432:.
422:34
420:.
397:.
389:.
381:.
371:40
369:.
355:^
341:.
333:.
325:.
317:.
305:.
293:^
279:.
271:.
259:.
229:,
217:,
203:^
154:.
111:sp
507:.
479::
471::
465:1
448:.
436::
428::
405:.
385::
377::
349:.
321::
313::
287:.
267::
225::
136:3
120:3
115:3
100:3
96:3
80:3
65:3
61:3
57:3
53:3
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.