2108:. BIFs only form if the water is allowed to supersaturate in dissolved iron (Fe) meaning there cannot be free oxygen or sulfur in the water column because it would form Fe (rust) or pyrite and precipitate out of solution. Following this supersaturation, the water must become oxygenated in order for the ferric rich bands to precipitate it must still be sulfur poor otherwise pyrite would form instead of Fe. It has been hypothesized that BIFs formed during the initial evolution of photosynthetic organisms that had phases of population growth, causing over production of oxygen. Due to this over production they would poison themselves causing a mass die off, which would cut off the source of oxygen and produce a large amount of CO
1630:
1480:
1468:
2089:", when redox conditions on Earth's surface are thought by most workers to have shifted fundamentally from reducing to oxidizing. This shift would have led to an incredible increase in sulfate weathering which would have led to an increase in sulfate in the oceans. The large isotopic fractionations that would likely be associated with bacteria reduction are produced for the first time. Although there was a distinct rise in seawater sulfate at this time it was likely still only less than 5–15% of present-day levels.
1577:
sulfate. Such reactions are known to occur by microbial processes but it is generally accepted that TSR is responsible for the bulk of these reactions, especially in deep or hot reservoirs. Thus, TSR occurs in deep reservoirs where the temperatures are much higher. BSR is geologically instantaneous in most geologic settings, while TSR occurs at rates in the order of hundreds of thousands of years. Although much slower than BSR, even TSR appears to be a geologically fairly fast process.
2161:
Since different sulfate sources within the ocean have distinct oxygen isotopic values it may be possible to use oxygen to trace the sulfur cycle. Biological sulfate reduction preferentially selects lighter oxygen isotopes for the same reason that lighter sulfur isotopes are preferred. By studying oxygen isotopes in ocean sediments over the last 10 million years were able to better constrain the sulfur concentrations in sea water through that same time. They found that the
122:
5446:
696:
1459:, and is the major biogenic gas emitted from the sea, where it is responsible for the distinctive “smell of the sea” along coastlines. DMS is the largest natural source of sulfur gas, but still only has a residence time of about one day in the atmosphere and a majority of it is redeposited in the oceans rather than making it to land. However, it is a significant factor in the climate system, as it is involved in the formation of clouds.
25:
148:
1905:
2010:
and thus this process determines if the organic matter is assimilated or buried. Sulfurization increases molecular weight and introduces a new moiety to the organic molecule which may inhibit its recognition by catabolic enzymes that degrade organic matter. Microbial ability for desulfurization is reflected by the presence of
2120:
also marks the first large scale sedimentary exhalative deposits showing a link between mineralization and a likely increase in the amount of sulfate in sea water. In the
Paleoproterozoic the sulfate in seawater had increased to an amount greater than in the Archean, but was still lower than present
1888:
is met by the anaerobic methanotrophic archaea in the SMTZ which oxidize it using sulfate as an electron acceptor. More sulfate is present at the SMTZ than methane. A 4:1 ratio of sulfate: methane is observed and the excess sulfate is directed towards organic matter degradation. Syntrophic aggregates
1776:
tube worms that grow around hydrothermal vents lack a digestive tract but contain specialized organelles called trophosomes within which autotrophic, sulfide oxidizing bacteria are housed. The tube worms provide the bacteria with sulfide and the bacteria shares the fixed carbon with the worms.
1616:
that use sulfide or elemental sulfur to fix carbon dioxide. The oxidation pathway includes the formation of various intermediate sulfur species, including elemental sulfur and thiosulfate. Under low oxygen concentrations, microbes will oxidize to elemental sulfur. This elemental sulfur accumulates as
2299:
exert an important control on the redox state of the metal-transporting fluids, and deposits can form from both oxidizing and reducing fluids. Metal-rich ore fluids tend to be, by necessity, comparatively sulfide deficient, so a substantial portion of the sulfide must be supplied from another source
2009:
Sulfurization of organic matter is a significant sulfur pool, containing 35-80% of the reduced sulfur in marine sediments. These organo-sulfur molecules are also desulfurized to release oxidized sulfur species like sulfite and sulfate. This desulfurization may allow degradation of the organic matter
1576:
deltas, and hydrothermal sediments which have intense microbial sulfate reduction because of the high concentration of dissolved sulfate in the seawater. Additionally, the high amounts of hydrogen sulfide found in oil and gas fields is thought to arise from the oxidation of petroleum hydrocarbons by
1555:
These processes occur because there are two very different thermal regimes in which sulfate is reduced, particularly in low-temperature and high-temperature environments. BSR usually occurs at lower temperatures from 0−80 °C, while TSR happens at much higher temperatures around 100–140 °C.
1859:
Throughout geologic history the sulfur cycle and the isotopic ratios have coevolved with the biosphere becoming overall more negative with the increases in biologically driven sulfate reduction, but also show substantial positive excursion. In general positive excursions in the sulfur isotopes mean
2272:
generation as long as the respective transition or base metals are present or transported to a sulfate reduction site. If the system runs out of reactive hydrocarbons, economically viable elemental sulfur deposits may form. Sulfur also acts as a reducing agent in many natural gas reservoirs, and
2125:
also act as proxies for atmospheric oxygen because sulfate is produced mostly through weathering of the continents in the presence of oxygen. The low levels in the
Proterozoic simply imply that levels of atmospheric oxygen fell between the abundances of the Phanerozoic and the deficiencies of the
2029:
of sulfur) represents the total outgassing of sulfur through geologic time. Rocks analyzed for sulfur content are generally organic-rich shales meaning they are likely controlled by biogenic sulfur reduction. Average seawater curves are generated from evaporites deposited throughout geologic time
1657:
fueled by sulfide oxidation. Some PSB can also perform aerobic sulfide oxidation in the presence of oxygen and can even grow chemoautotrophically under low light conditions. GSB lack this metabolic potential and have compensated by developing efficient light harvesting systems. PSB can be found in
1921:
utilizes multiple oxidants because the concentrations of the electron acceptors are depth dependent. In the upper sediment layers oxygen and nitrate are the preferred oxidants because of the high energy yield from the reaction, and in the suboxic zones iron and manganese take on the role. Sulfide
1855:
of sulfur intermediates in the sediment. This view has changed since the 2010s that sulfate reduction can fractionate to 66 permil. As substrates for disproportionation are limited by the product of sulfate reduction, the isotopic effect of disproportionation should be less than 16 permil in most
1556:
Temperatures for TSR are not as well defined; the lowest confirmed temperature is 127 °C and the highest temperatures occur in settings around 160−180 °C. These two different regimes appear because at higher temperatures most sulfate-reducing microbes can no longer metabolize due to the
2160:
Over a shorter time scale (ten million years) changes in the sulfur cycle are easier to observe and can be even better constrained with oxygen isotopes. Oxygen is continually incorporated into the sulfur cycle through sulfate oxidation and then released when that sulfate is reduced once again.
2315:
or precious metals are discovered and either burned or milled, sulfur becomes a waste product that must be dealt with properly, or it can become a pollutant. The burning of fossil fuels has greatly increased the amount of sulfur in our present-day atmosphere. Sulfur acts as a pollutant and an
1916:
or used as electron donor or to sulfurize organic matter by microbes. Pyrite is formed through two pathways: the polysulfide and the hydrogen sulfide pathway. The polysulfide pathway is dominant until the depletion of elemental sulfur since elemental sulfur is necessary in the formation of
2141:
high carbon burial rates increased the atmospheric oxygen level to >10% of its present-day value. In the Latest
Neoproterozoic another major oxidizing event occurred on Earth's surface that resulted in an oxic deep ocean and possibly allowed for the appearance of multicellular life.
2045:(4.6–2.5 Ga) most systems appeared to be sulfate-limited. Some small Archean evaporite deposits require that at least locally elevated concentrations (possibly due to local volcanic activity) of sulfate existed in order for them to be supersaturated and precipitate out of solution.
1835:
should be the same as the overall isotope ratio in the water column at their time of precipitation. Sulfate reduction through biologic activity strongly differentiates between the two isotopes because of the more rapid enzymic reaction with S. Average present day seawater values of
1922:
oxidation yields various sulfur intermediates such as elemental sulfur, thiosulfate, sulfite, and sulfate.The sulfur intermediates formed during sulfide oxidation are unique to this process and thus are indicative of sulfide oxidation when found in environmental samples. Sulfur
2365:) in the global cycle, at the expense of the storage of reduced sulfur in the Earth's crust. Therefore, human activities do not cause a major change in the global pools of sulfur, but they do produce massive changes in the annual flux of sulfur through the atmosphere.
2352:
Although the sulfur curve shows shifts between net sulfur oxidation and net sulfur reduction in the geologic past, the magnitude of the current human impact is probably unprecedented in the geologic record. Human activities greatly increase the flux of sulfur to the
2074:
2.8 Ga marks the first evidence for oxygen production through photosynthesis. This is important because there cannot be sulfur oxidation without oxygen in the atmosphere. This exemplifies the coevolution of the oxygen and sulfur cycles as well as the biosphere.
1658:
various environments ranging from hot sulfur springs and alkaline lakes to wastewater treatment plants. GSB populate stratified lakes with high reduced sulfur concentrations and can even grow in hydrothermal vents by using infra-red light to perform photosynthesis.
2393:, however, the average rain pH is between 4.2 and 4.4. Since pH is on a log scale dropping by 1 (the difference between normal rain water and acid rain) has a dramatic effect on the strength of the acid. In the United States, roughly two thirds of all SO
1621:
form long chains that span the length between oxic and sulfidic zones of the coastal sediments. The bacteria present in the sulfide rich zones oxidize the sulfide and transport the electrons to the bacteria present in the oxygen rich zone through multiple
2384:
is a broad term referring to a mixture of wet and dry deposition (deposited material) from the atmosphere containing higher than normal amounts of nitric and sulfuric acids. Distilled water (water without any dissolved constituents), which contains no
2041:. All sulfur in the atmosphere would be released during volcanic eruptions. When the oceans condensed on Earth, the atmosphere was essentially swept clean of sulfur gases, owing to their high solubility in water. Throughout the majority of the
2112:
through the decomposition of their bodies, allowing for another bacterial bloom. After 1.8 Ga sulfate concentrations were sufficient to increase rates of sulfate reduction to greater than the delivery flux of iron to the oceans.
2176:
cycles changed the area of continental shelves which then disrupted the sulfur processing, lowering the concentration of sulfate in the sea water. This was a drastic change as compared to preglacial times before 2 million years ago.
1796:
and of geochemical importance. Of those four, two (S, light and S, heavy) comprise (99.22%) of sulfur on Earth. The vast majority (95.02%) of sulfur occurs as S with only 4.21% in S. The ratio of these two isotopes is fixed in the
3470:
Wilbanks, Elizabeth G.; Jaekel, Ulrike; Salman, Verena; Humphrey, Parris T.; Eisen, Jonathan A.; Facciotti, Marc T.; Buckley, Daniel H.; Zinder, Stephen H.; Druschel, Gregory K.; Fike, David A.; Orphan, Victoria J. (November 2014).
2226:
exceeded 10 present atmospheric level after the Great
Oxygenation Event. Oxygen played an essential role in the global sulfur cycles after the Great Oxygenation Event, such as oxidative weathering of sulfides. The burial of
2205:
S value according to the mass dependent fractionation law. The Great
Oxidation Event represented a massive transition of global sulfur cycles. Before the Great Oxidation Event, the sulfur cycle was heavily influenced by the
1524:, elemental sulfur and metal sulfides. However, the reactive organic compounds differ for BSR and TSR because of the mutually exclusive temperature regimes. Organic acids are the main organic reactants for BSR and branched/
2336:
has greatly increased the amount of sulfur in the atmosphere and ocean and depleted the sedimentary rock sink. Without human impact sulfur would stay tied up in rocks for millions of years until it was uplifted through
1567:
BSR and TSR occur at different depths. BSR takes place in low-temperature environments, which are shallower settings such as oil and gas fields. BSR can also take place in modern marine sedimentary environments such as
1411:
The primary natural source of sulfur to the atmosphere is sea spray or windblown sulfur-rich dust, neither of which is long lived in the atmosphere. In recent times, the large annual input of sulfur from the burning of
3152:
Bjerg, Jesper T.; Boschker, Henricus T. S.; Larsen, Steffen; Berry, David; Schmid, Markus; Millo, Diego; Tataru, Paula; Meysman, Filip J. R.; Wagner, Michael; Nielsen, Lars Peter; Schramm, Andreas (2018-05-29).
1436:
have caused large scale burning of these measures, and consequential release of sulfur to the atmosphere. This has led to substantial disruption to the climate system, and is one of the proposed causes of the
1969:
S and S). There are two major outputs of sulfur from the oceans. The first sink is the burial of sulfate either as marine evaporites (such as gypsum) or carbonate-associated sulfate (CAS), which accounts for
720:
2247:
and most metal deposits because it acts as an oxidizing or reducing agent. The vast majority of the major mineral deposits on Earth contain a substantial amount of sulfur including, but not limited to
1617:
sulfur globules, intracellularly or extracellularly, to be consumed under low sulfur concentrations. To ameliorate low oxidant concentrations (that is, to find an electron sink), sulfur oxidizers like
4778:
Konhauser KO, Lalonde SV, Planavsky NJ, Pecoits E, Lyons TW, Mojzsis SJ, et al. (October 2011). "Aerobic bacterial pyrite oxidation and acid rock drainage during the Great
Oxidation Event".
1757:
O) to the symbiont while the symbiont generates organic carbon for sustaining the metabolic activities of the host. The produced sulfate usually combines with the leached calcium ions to form
4457:
John EH, Wignall PB, Newton RJ, Bottrell SH (August 2010). "δ34SCAS and δ18OCAS records during the
Frasnian–Famennian (Late Devonian) transition and their bearing on mass extinction models".
2361:
at a rate that mobilizes 150 x 10 gS/yr, which is more than double the rate of 100 years ago. The result of human impact on these processes is to increase the pool of oxidized sulfur (SO
2214:, which induced the sulfur isotope mass-independent fractionation (ΔS ≠ 0). The preservation of sulfur isotope mass-independent fractionation signals requires the atmospheric O
4322:
Lyons TW, Gellatly AM, McGoldrick PJ, Kah LC (2006). "Proterozoic sedimentary exhalative (SEDEX) deposits and links to evolving global ocean chemistry". In Kesler SE, Ohmoto H (eds.).
1497:
pathway, sulfate can be reduced either bacterially (bacterial sulfate reduction) or inorganically (thermochemical sulfate reduction). This pathway involves the reduction of sulfate by
4492:
Newton RJ, Pevitt EL, Wignall PB, Bottrell SH (February 2004). "Large shifts in the isotopic composition of seawater sulphate across the Permo–Triassic boundary in northern Italy".
2885:
Krouse HR, Viau CA, Eliuk LS, Ueda A, Halas S (1988). "Chemical and isotopic evidence of thermochemical sulphate reduction by light hydrocarbon gases in deep carbonate reservoirs".
2389:, has a neutral pH of 7. Rain naturally has a slightly acidic pH of 5.6, because carbon dioxide and water in the air react together to form carbonic acid, a very weak acid. Around
2349:
processes. Instead it is being drilled, pumped and burned at a steadily increasing rate. Over the most polluted areas there has been a 30-fold increase in sulfate deposition.
2288:
states determine whether sulfides will precipitate. Most sulfide brines will remain in concentration until they reach reducing conditions, a higher pH, or lower temperatures.
3766:"Methane-Fueled Syntrophy through Extracellular Electron Transfer: Uncovering the Genomic Traits Conserved within Diverse Bacterial Partners of Anaerobic Methanotrophic Archaea"
776:
cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Steps of the
2408:, sulfur is increasingly used as a component of fertilizers. Recently sulfur deficiency has become widespread in many countries in Europe. Because of actions taken to limit
1827:
Formation of sulfur minerals through non-biogenic processes does not substantially differentiate between the light and heavy isotopes, therefore sulfur isotope ratios in
2006:
which matches the input fluxes, implying the modern marine sulfur budget is at steady state. The residence time of sulfur in modern global oceans is 13,000,000 years.
4864:
Pham M, Müller JF, Brasseur GP, Granier C, Mégie G (May 1996). "A 3D model study of the global sulphur cycle: Contributions of anthropogenic and biogenic sources".
1596:
S in any deep reservoir, then it is assumed that TSR has taken over. This is due to the fact that thermal cracking of hydrocarbons doesn't provide more than 3% of H
4163:
Wegner, Carl-Eric; Richter-Heitmann, Tim; Klindworth, Anna; Klockow, Christine; Richter, Michael; Achstetter, Tilman; Glöckner, Frank Oliver; Harder, Jens (2013).
1103:. Thus, elemental sulfur can either give or receive electrons depending on its environment. On the anoxic early Earth, most sulfur was present in minerals such as
2218:
lower than 10 of present atmospheric level (PAL). The disappearance of sulfur isotope mass-independent fractionation at ~2.45 Ga indicates that atmospheric
2030:
because again, since they do not discriminate between the heavy and light sulfur isotopes, they should mimic the ocean composition at the time of deposition.
727:
2308:
are generally consistent with a seawater sulfate source, suggesting baryte formation by reaction between hydrothermal barium and sulfate in ambient seawater.
2807:
Aharon P, Fu B (2000). "Microbial sulfate reduction rates and sulfur and oxygen isotope fractionations at oil and gas seeps in deepwater Gulf of Mexico".
2412:
atmospheric inputs of sulfur continue to decrease, As a result, the deficit in the sulfur input is likely to increase unless sulfur fertilizers are used.
1604:
S is affected by several factors such as, the availability of organic reactants and sulfate and the presence/availability of base and transition metals.
1612:
Sulfide oxidation is performed by both bacteria and archaea in a variety of environmental conditions. Aerobic sulfide oxidation is usually performed by
968:
Sulfur has four main oxidation states in nature, which are −2, +2, +4, and +6. The common sulfur species of each oxidation state are listed as follows:
3764:
Skennerton, Connor T.; Chourey, Karuna; Iyer, Ramsunder; Hettich, Robert L.; Tyson, Gene W.; Orphan, Victoria J. (2017-09-06). Dubilier, Nicole (ed.).
2600:
Reheis MC, Kihl R (May 1995). "Dust deposition in southern Nevada and
California, 1984–1989: Relations to climate, source area, and source lithology".
2280:. The presence or absence of sulfur is one of the limiting factors in the concentration of precious metals and their precipitation from solution.
1504:
The main products and reactants of bacterial sulfate reduction (BSR) and thermochemical sulfate reduction (TSR) are very similar. For both, various
4899:
Brimblecombe P, Hammer C, Rodhe H, Ryaboshapko A, Boutron CF (1989). "Human
Influences on the sulphur cycle.". In Brimblecombe P, Lein AY (eds.).
2764:
Jørgensen BB, Isaksen MF, Jannasch HW (December 1992). "Bacterial
Sulfate Reduction Above 100{degrees}C in Deep-Sea Hydrothermal Vent Sediments".
5088:
2193:(MIF) in the sedimentary records at around 2.45 billion years ago (Ga). The MIF of sulfur isotope (ΔS) is defined by the deviation of measured
5125:
2052:
rocks from this time still have an isotopic value of 0 because the biosphere was not developed enough (possibly at all) to fractionate sulfur.
1868:
The marine sulfur cycle is driven by sulfate reduction because hydrogen sulfide is oxidized by microbes for energy or is oxidized abiotically.
2063:
S is still basically 0. Shortly after, at 3.4 Ga the first evidence for minimal fractionation in evaporitic sulfate in association with
2276:
Important sources of sulfur in ore deposits are generally deep-seated, but they can also come from local country rocks, seawater, or marine
1745:
are primary sulfur oxidizing bacteria, and form chemosynthetic symbioses with animal hosts. The host provides metabolic substrates (e.g., CO
1801:
and has been since its formation. The bulk Earth sulfur isotopic ratio is thought to be the same as the ratio of 22.22 measured from the
1484:
5093:
1961:
S = +6‰) is the primary input of sulfur to the oceans. Other sources are metamorphic and volcanic degassing and hydrothermal activity (
3329:
Sievert SM, Hügler M, Taylor CD, Wirsen CO (2008). "Sulfur Oxidation at Deep-Sea Hydrothermal Vents". In Dahl C, Friedrich CG (eds.).
5360:
4527:
Gill BC, Lyons TW, Jenkyns HC (December 2011). "A global perturbation to the sulfur cycle during the Toarcian Oceanic Anoxic Event".
243:
236:
3222:"Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea"
5047:
2644:
Machel HG, Krouse HR, Sassen R (1995). "Products and distinguishing criteria of bacterial and thermochemical sulfate reduction".
2253:
1665:
2496:
Bickle MJ, Alt JC, Teagle DA (1994). "Sulfur transport and sulphur isotope fractionations in ocean floor hydrothermal systems".
4041:
4908:
4829:
Berner RA, Raiswell R (1983). "Burial of organic carbon and pyrite sulfur in sediments over phanerozoic time: a new theory".
4331:
3346:
2480:
1438:
713:
1897:
have been discovered and the underlying mechanisms observed include direct interspecies electron transfer using large multi
5292:
1957:
with the sulfur isotope composition of ~3‰. Riverine sulfate derived from the terrestrial weathering of sulfide minerals (
679:
89:
3862:"Mass-dependent sulfur isotope fractionation during reoxidative sulfur cycling: A case study from Mangrove Lake, Bermuda"
2048:
3.8–3.6 Ga marks the beginning of the exposed geologic record because this is the age of the oldest rocks on Earth.
1668:
that oxidize hydrogen sulfide with oxygen to produce elemental sulfur or sulfate. The chemical reactions are as follows:
204:
61:
2137:
episodes where the entire globe including the oceans was covered in a layer of ice cutting off oxygenation. In the late
2059:
is established and provides a weak source of sulfate to the global ocean with sulfate concentrations incredibly low the
5396:
5272:
5118:
1824:. Positive values correlate to increased levels of S, whereas negative values correlate with greater S in a sample.
1768:
with other microbes, and even animals. PSB and sulfate reducers form microbial aggregates called “pink berries” in the
655:
275:
3221:
3927:
3589:
3546:
2740:
108:
2022:
The isotopic composition of sedimentary sulfides provides primary information on the evolution of the sulfur cycle.
68:
2688:
Machel HG (2001). "Bacterial and thermochemical sulfate reduction in diagenetic settings — old and new insights".
5421:
5002:
Ceccotti SP (1996). "Plant nutrient sulphur—a review of nutrient balance, environmental impact and fertilizers".
2436:
2295:
under elevated thermal conditions, typically in extensional tectonic settings. The redox conditions of the basin
2249:
1881:
1869:
1494:
1472:
312:
4165:"Expression of sulfatases in Rhodopirellula baltica and the diversity of sulfatases in the genus Rhodopirellula"
2190:
2100:; their disappearance marks a distinct shift in the chemistry of ocean water. BIFs have alternating layers of
1890:
1873:
1848:
1844:
1573:
1353:
is assimilated by organisms, it is reduced and converted to organic sulfur, which is an essential component of
46:
3860:
Pellerin, André; Bui, Thi Hao; Rough, Mikaella; Mucci, Alfonso; Canfield, Donald E.; Wing, Boswell A. (2015).
75:
5490:
5449:
5111:
4348:
1918:
700:
42:
3670:"Estimating the effect of elemental sulfur disproportionation on the sulfur-isotope signatures in sediments"
3267:
Kushkevych, Ivan; Procházka, Jiří; Gajdács, Márió; Rittmann, Simon K.-M. R.; Vítězová, Monika (2021-06-15).
5401:
5256:
5155:
4924:
Zhao F, Hawkesford M, McGrath SP (1999). "Sulphur Assimilation and Effects on Yield and Quality of Wheat".
2067:
derived sulfides can be seen in the rock record. This fractionation shows possible evidence for anoxygenic
1390:
380:
372:
358:
285:
5431:
4658:"Sulfur isotopes track the global extent and dynamics of euxinia during Cretaceous Oceanic Anoxic Event 2"
1926:
of these intermediates and other sulfur species has been a useful tool in the study of sulfide oxidation.
635:
3821:
Yücel, Mustafa; Konovalov, Sergey K.; Moore, Tommy S.; Janzen, Christopher P.; Luther, George W. (2010).
1557:
57:
1860:
that there is an excess of pyrite deposition rather than oxidation of sulfide minerals exposed on land.
4727:
Farquhar J, Bao H, Thiemens M (August 2000). "Atmospheric influence of Earth's earliest sulfur cycle".
2979:
Jørgensen BB (1982). "Mineralization of organic matter in the sea bed—the role of sulphate reduction".
2584:
2085:
2.3 Ga sulfate increases to more than 1 mM; this increase in sulfate is coincident with the "
1772:
of Massachusetts within which sulfur cycling occurs through the direct exchange of sulfur species. The
1448:
3473:"Microscale sulfur cycling in the phototrophic pink berry consortia of the S ippewissett S alt M arsh"
2376:
through reactions with water in the atmosphere. Once the acid is completely dissociated in water the
3413:
Pedersen RB, Rapp HT, Thorseth IH, Lilley MD, Barriga FJ, Baumberger T, et al. (November 2010).
2380:
can drop to 4.3 or lower causing damage to both man-made and natural systems. According to the EPA,
1802:
1654:
956:
3030:
Holmer M, Storkholm P (2001). "Sulphate reduction and sulphur cycling in lake sediments: a review".
2273:
generally, ore-forming fluids have a close relationship with ancient hydrocarbon seeps or vents.
2129:
750 million years ago (Ma) there is a renewed deposition of BIF which marks a significant change in
5389:
5384:
2357:, some of which is transported globally. Humans are mining coal and extracting petroleum from the
448:
443:
199:
4213:
Johnston DT (2011). "Multiple sulfur isotopes and the evolution of Earth's surface sulfur cycle".
1629:
772:, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global
5480:
5323:
4164:
3822:
3669:
2304:
S-containing) water column is a necessary source of that sulfide. When present, the δS values of
2300:
at the site of mineralization. Bacterial reduction of seawater sulfate or a euxinic (anoxic and H
2186:
2086:
1912:
Sulfide produced by sulfate reduction can be oxidized by iron minerals to make iron sulfides and
769:
602:
35:
1904:
5212:
5043:
4656:
Owens JD, Gill BC, Jenkyns HC, Bates SM, Severmann S, Kuypers MM, et al. (November 2013).
4324:
Evolution of Early Earth's Atmosphere, Hydrosphere, and Biosphere—Constraints from Ore Deposits
2936:
Muyzer G, Stams AJ (June 2008). "The ecology and biotechnology of sulphate-reducing bacteria".
2211:
2093:
2038:
1650:
1584:
are key processes in the oceanic sulfur cycle. Approximately, 10% (of the total gas) of H
785:
552:
2180:
5475:
5416:
1923:
1851:
up to 46 permil and fractionation larger than 46 permil recorded in sediments must be due to
1646:
911:
S, oxidation state = –2). An analogous process for organic nitrogen compounds is deamination.
295:
5067:
4540:
4505:
1560:
of proteins or deactivation of enzymes, so TSR takes over. However, in hot sediments around
1532:
are the main organic reactants for TSR. The inorganic reaction products in BSR and TSR are H
1479:
5335:
5165:
5142:
5134:
4968:
4873:
4838:
4787:
4736:
4669:
4606:
4536:
4501:
4466:
4415:
4360:
4264:
4222:
4176:
4111:
4053:
4012:
3963:
3873:
3834:
3720:
3618:
3484:
3426:
3166:
3039:
2988:
2894:
2816:
2697:
2653:
2609:
2544:
2505:
2426:
2082:
to have a depleted δ S which provide the first compelling evidence for sulfate reduction.
2049:
745:
464:
216:
209:
139:
82:
8:
5470:
5426:
5367:
1508:
and dissolved sulfate are the reactants, and the products or by-products are as follows:
1068:
870:
832:
Incorporation of sulfide into organic compounds (including metal-containing derivatives).
623:
433:
324:
4972:
4877:
4842:
4791:
4740:
4673:
4610:
4470:
4419:
4364:
4268:
4226:
4180:
4115:
4057:
4016:
3976:
3967:
3951:
3877:
3838:
3724:
3622:
3488:
3430:
3170:
3043:
2992:
2898:
2820:
2701:
2657:
2613:
2548:
2509:
5019:
4984:
4811:
4760:
4692:
4657:
4638:
4439:
4384:
4292:"Isotopic inferences of ancient biochemistries-Carbon, sulfur, hydrogen, and nitrogen."
4140:
4099:
4077:
3981:
3919:
3907:
3798:
3765:
3689:
3650:
3523:
3472:
3447:
3414:
3390:
3363:
3303:
3268:
3197:
3154:
3116:
3081:
3012:
2961:
2918:
2867:
2789:
2713:
1983:
1930:
1876:(AOM) both of which produce carbon dioxide. At depths where sulfate is depleted,
1852:
1789:
1386:
835:
562:
5037:
4513:
3707:
Egger, Matthias; Riedinger, Natascha; Mogollón, José M.; Jørgensen, Bo Barker (2018).
3606:
2828:
2709:
2586:
Oxygen isotopes in marine sulfate and the sulfur cycle over the last 140 million years
5411:
5379:
5313:
5160:
4904:
4885:
4850:
4815:
4803:
4752:
4697:
4630:
4622:
4579:
4562:
Paytan, A. (1998-11-20). "Sulfur Isotopic Composition of Cenozoic Seawater Sulfate".
4431:
4376:
4327:
4192:
4145:
4127:
4069:
3985:
3923:
3889:
3803:
3785:
3746:
3693:
3642:
3634:
3585:
3528:
3510:
3452:
3415:"Discovery of a black smoker vent field and vent fauna at the Arctic Mid-Ocean Ridge"
3395:
3342:
3308:
3290:
3249:
3241:
3237:
3202:
3184:
3121:
3103:
3055:
3051:
3004:
2953:
2910:
2859:
2793:
2781:
2746:
2736:
2665:
2562:
2476:
2431:
2421:
2292:
1661:
1561:
1004:
757:
592:
438:
290:
226:
5023:
4988:
4764:
4642:
4388:
4234:
3708:
2965:
2871:
2401:
come from electric power generation that relies on burning fossil fuels, like coal.
2291:
Ore fluids are generally linked to metal-rich waters that have been heated within a
2037:
S value of 0. Since there was no biologic activity on early Earth there would be no
1361:
does not act as a major sink for sulfur, instead the majority of sulfur is found in
5330:
5318:
5249:
5219:
5011:
4976:
4933:
4881:
4846:
4795:
4744:
4687:
4677:
4614:
4597:
Paytan, A. (2004-06-11). "Seawater Sulfur Isotope Fluctuations in the Cretaceous".
4571:
4544:
4509:
4474:
4443:
4423:
4368:
4272:
4230:
4184:
4135:
4119:
4081:
4061:
4020:
3971:
3915:
3881:
3842:
3793:
3777:
3736:
3728:
3681:
3654:
3626:
3518:
3500:
3492:
3442:
3434:
3385:
3375:
3334:
3298:
3280:
3233:
3192:
3174:
3111:
3093:
3047:
3016:
2996:
2945:
2922:
2902:
2851:
2824:
2773:
2717:
2705:
2661:
2617:
2552:
2513:
2390:
2269:
2162:
2117:
2097:
2079:
1509:
1505:
1498:
1366:
980:
972:
904:
855:
797:
789:
609:
597:
307:
170:
4951:
Blake-Kalff MM (2000). "Diagnosing sulfur deficiency in field-grown oilseed rape (
4575:
4478:
4372:
3846:
3685:
2777:
1467:
5485:
5350:
5308:
4748:
4291:
4188:
3578:
3338:
3269:"Molecular Physiology of Anaerobic Phototrophic Purple and Green Sulfur Bacteria"
2517:
2405:
2358:
2324:
Human activities have a major effect on the global sulfur cycle. The burning of
2257:
2130:
1444:
1212:
1136:
1120:
1082:
1061:
1013:
930:
587:
542:
406:
389:
2842:
Goldstein TP, Aizenshtat Z (1994). "Thermochemical sulfate reduction a review".
1809:. That ratio is accepted as the international standard and is therefore set at
1569:
5229:
5202:
5197:
5177:
5172:
4662:
Proceedings of the National Academy of Sciences of the United States of America
4548:
3668:
Tsang, Man-Yin; Böttcher, Michael Ernst; Wortmann, Ulrich Georg (August 2023).
2386:
2138:
2134:
2056:
1877:
1793:
1618:
992:
922:
614:
557:
512:
502:
497:
472:
411:
394:
270:
265:
257:
4980:
3885:
3732:
1982:
S = +21‰). The second sulfur sink is pyrite burial in shelf sediments or deep
1929:
The sulfur cycle in marine environments has been well-studied via the tool of
5464:
5406:
5192:
5187:
4626:
4403:
4131:
4042:"Low marine sulphate and protracted oxygenation of the Proterozoic biosphere"
3893:
3789:
3750:
3638:
3514:
3380:
3294:
3245:
3188:
3107:
3098:
3059:
3008:
2914:
2863:
2373:
2153:
S, with an average value close to that of today. Notably changes in seawater
1462:
1452:
1433:
1425:
1050:
547:
537:
492:
487:
477:
425:
348:
280:
5098:
4682:
4618:
4402:
Gill BC, Lyons TW, Young SA, Kump LR, Knoll AH, Saltzman MR (January 2011).
4276:
4123:
4100:"The life sulfuric: microbial ecology of sulfur cycling in marine sediments"
3861:
3630:
3496:
3179:
2750:
2557:
2532:
2025:
The total inventory of sulfur compounds on the surface of the Earth (nearly
1404:
sulfate reduction and sulfide re-oxidation on continental shelves and slopes
903:
in which organic molecules containing sulfur can be desulfurized, producing
5234:
5207:
5150:
4937:
4807:
4756:
4701:
4634:
4435:
4196:
4149:
4073:
4025:
4000:
3807:
3646:
3532:
3456:
3399:
3312:
3253:
3206:
3125:
2957:
2785:
2566:
2312:
2181:
The Great Oxidation Event and sulfur isotope mass-independent fractionation
1894:
1798:
1773:
522:
507:
353:
302:
194:
186:
5089:
Sulfur Oxidation from Soil Microbiology course at Virginia Tech University
4583:
4380:
3781:
1761:, which can form widespread deposits on near mid-ocean spreading centers.
5355:
5345:
5340:
5244:
4404:"Geochemical evidence for widespread euxinia in the later Cambrian ocean"
4326:. Geological Society of America Memoir. Vol. 198. pp. 169–184.
3285:
2333:
2329:
2244:
2207:
2170:
2122:
1456:
1417:
1276:
1260:
1228:
934:
847:
482:
399:
231:
221:
165:
157:
121:
5103:
4799:
4427:
4065:
3607:"Large Sulfur Isotope Fractionation Does Not Require Disproportionation"
2949:
5224:
5182:
5015:
4162:
3741:
3438:
3155:"Long-distance electron transport in individual, living cable bacteria"
2855:
2533:"Reducing food's environmental impacts through producers and consumers"
2354:
2346:
2265:
2101:
2068:
1885:
1769:
1642:
1638:
1592:
S is produced in TSR settings. If there is more than a few percent of H
1116:
894:
567:
532:
527:
517:
340:
2621:
752:
moves between rocks, waterways and living systems. It is important in
5372:
5277:
4255:
Canfield DE, Raiswell R (1999). "The evolution of the sulfur cycle".
3505:
3000:
2906:
2409:
2381:
2296:
2277:
2011:
1806:
1765:
1623:
1613:
1581:
1521:
1378:
1374:
1358:
1292:
1144:
1112:
1111:). Over Earth history, the amount of mobile sulfur increased through
803:
660:
628:
1123:
in an oxygenated atmosphere. Earth's main sulfur sink is the oceans
24:
2338:
2166:
2157:
S occurred during extinction and climatic events during this time.
1362:
926:
147:
4898:
3266:
3080:
Jørgensen, Bo Barker; Findlay, Alyssa J.; Pellerin, André (2019).
2033:
4.6 billion years ago (Ga) the Earth formed and had a theoretical
1917:
polysulfides, then the hydrogen sulfide pathway takes over.
897:. The oxidation states of sulfur are +6 in sulfate and –2 in R–SH.
2342:
2173:
2042:
1821:
1429:
1394:
1354:
1244:
1100:
1086:
1020:
851:
843:
811:
807:
765:
753:
4777:
1814:
4347:
Hoffman PF, Kaufman AJ, Halverson GP, Schrag DP (August 1998).
4098:
Wasmund, Kenneth; Mußmann, Marc; Loy, Alexander (August 2017).
3706:
2446:
2305:
2261:
2228:
1913:
1832:
1828:
1758:
1529:
1382:
1180:
1104:
998:
839:
773:
761:
749:
2231:
in sediments in turn contributes to the accumulation of free O
2189:(GOE) is characterized by the disappearance of sulfur isotope
2096:(BIF) are common sedimentary rocks throughout the Archean and
1908:
Oxidant concentrations at different depths in marine sediments
5282:
4346:
2441:
2285:
2105:
2064:
1501:
to produce hydrogen sulfide, which occurs in both processes.
1370:
949:
in which elemental sulfur can be reduced to hydrogen sulfide.
890:
886:
756:
as it affects many minerals and in life because sulfur is an
669:
3763:
3469:
1813: = 0.00. Deviation from 0.00 is expressed as the
1147:(air pollution) of different foods per 100 grams of protein
5287:
4491:
2325:
1898:
1413:
1324:
1308:
1196:
1164:
674:
4321:
3412:
1872:
is driven by the degradation of buried organic matter and
1664:
emit hydrogen sulfide that support the carbon fixation of
1463:
Biologically and thermochemically driven sulfate reduction
4863:
4456:
3820:
3328:
3709:"Global diffusive fluxes of methane in marine sediments"
3547:"Understanding the symbiosis between the giant tubeworm
3151:
3079:
2763:
1965:
S = 0‰), which release reduced sulfur species (such as H
1764:
Sulfur metabolizing microbes are often engaged in close
3914:. Vol. 10. Amsterdam: Elsevier. pp. 559–591.
3584:(2nd ed.). San Diego, California: Academic Press.
2377:
2281:
5083:
4289:
1397:
in the oceans is controlled by three major processes:
4923:
4655:
4001:"The evolution of the Earth surface sulfur reservoir"
3859:
3667:
3605:
Sim, Min Sub; Bosak, Tanja; Ono, Shuhei (July 2011).
3082:"The Biogeochemical Sulfur Cycle of Marine Sediments"
5070:. Sulphurinstitute.org. Retrieved on 16 August 2012.
4901:
Evolution of the Global Biogeochemical Sulphur Cycle
4298:. Princeton, New Jersey: Princeton University Press.
3823:"Sulfur speciation in the upper Black Sea sediments"
3142:(6th ed). CRC Press. pp. 479-516. ISBN 9781466592414
2116:
Along with the disappearance of BIF, the end of the
1407:
burial of anhydrite and pyrite in the oceanic crust.
921:), oxidation state = 0. This reaction occurs in the
4726:
2884:
2841:
2243:Sulfur is intimately involved in the production of
1998:S = −20‰). The total marine sulfur output flux is
1937:S. The modern global oceans have sulfur storage of
1588:
S is produced in BSR settings, whereas 90% of the H
49:. Unsourced material may be challenged and removed.
4401:
3577:
2643:
1564:BSR can happen at temperatures up to 110 °C.
4097:
3361:
1884:(SMTZ), the upwelling of methane produced by the
1637:Anaerobic sulfide oxidation is performed by both
5462:
4722:
4720:
4526:
4254:
4250:
4248:
4246:
4244:
4208:
4206:
3333:. Springer Berlin Heidelberg. pp. 238–258.
2017:
3949:
3324:
3322:
3220:Ghosh, Wriddhiman; Dam, Bomba (November 2009).
3159:Proceedings of the National Academy of Sciences
3029:
2495:
1633:Biogeochemical sulfur cycle of marine sediments
4828:
3571:
3569:
3567:
3565:
3563:
3551:and chemoautotrophic sulfur-oxidizing bacteria
2524:
2470:
2149:has generally varied between +10‰ and +30‰ in
2145:During the last 600 million years, seawater SO
5119:
5030:
4717:
4317:
4315:
4313:
4311:
4309:
4307:
4305:
4241:
4203:
4039:
3956:Annual Review of Earth and Planetary Sciences
2683:
2681:
2679:
2677:
2675:
2639:
2637:
2635:
2633:
2631:
1607:
721:
3945:
3943:
3941:
3939:
3906:
3580:Biogeochemistry an analysis of global change
3319:
2602:Journal of Geophysical Research: Atmospheres
2530:
1949:S value of +21‰. The overall input flux is
1580:BSR in shallow environments and TSR in deep
4950:
4290:Schidlowski M, Hayes JM, Kaplan IR (1983).
4040:Kah LC, Lyons TW, Frank TD (October 2004).
3604:
3575:
3560:
3273:International Journal of Molecular Sciences
2935:
1076:
933:. Often the elemental sulfur is stored as
5126:
5112:
5094:Sulfur Cycle at Carnegie Mellon University
4302:
2672:
2628:
2599:
728:
714:
5133:
4691:
4681:
4139:
4024:
3975:
3936:
3797:
3740:
3522:
3504:
3446:
3389:
3379:
3302:
3284:
3196:
3178:
3115:
3097:
2978:
2578:
2576:
2556:
2372:is emitted as an air pollutant, it forms
2078:2.7–2.5 Ga is the age of the oldest
963:
109:Learn how and when to remove this message
5001:
4212:
3998:
3219:
2806:
1903:
1628:
1478:
1466:
120:
3362:Klotz MG, Bryant DA, Hanson TE (2011).
2582:
2475:(11th ed.). Pearson. p. 136.
2466:
2464:
2462:
2254:Carbonate-hosted lead-zinc ore deposits
2121:day values. The sulfate levels in the
1945:, mainly occurring as sulfate with the
1473:Dissimilatory sulfate reduction pathway
959:generate hydrogen sulfide from sulfate.
5463:
5050:from the original on December 13, 2011
4596:
4561:
3138:Fike, Bradley, Leavitt (Jan 1, 2015).
2730:
2687:
2573:
2238:
1863:
1488:(key intermediate in the sulfur cycle)
5107:
4093:
4091:
3952:"Rethinking the Ancient Sulfur Cycle"
3950:Fike DA, Bradley AS, Rose CV (2015).
3075:
3073:
3071:
3069:
1626:strings where the oxygen is reduced.
1485:3′-phosphoadenosine-5′-phosphosulfate
943:by sulfur oxidizers produces sulfate.
4903:. New York: Wiley. pp. 77–121.
2459:
2316:economic resource at the same time.
1843:Prior to 2010s, it was thought that
1447:is produced by the decomposition of
1420:has added a substantial amount of SO
47:adding citations to reliable sources
18:
4714:Tychyn et al. (2004) incomplete ref
4529:Earth and Planetary Science Letters
4494:Earth and Planetary Science Letters
3977:10.1146/annurev-earth-060313-054802
3910:(2014). "The global sulfur cycle".
2589:(PhD). Harvard University. 3174055.
2256:(Mississippi Valley-Type MVT), and
2201:S value inferred from the measured
862:These are often termed as follows:
636:Biogeochemical planetary boundaries
13:
5397:Human impact on the nitrogen cycle
4104:Environmental Microbiology Reports
4088:
3920:10.1016/B978-0-08-095975-7.00814-7
3066:
2210:(UV) radiation and the associated
1572:inland seas, continental shelves,
14:
5502:
5077:
4349:"A neoproterozoic snowball earth"
2341:events and then released through
1439:Permian–Triassic extinction event
796:S), elemental sulfur, as well as
5445:
5444:
3238:10.1111/j.1574-6976.2009.00187.x
3052:10.1046/j.1365-2427.2001.00687.x
2531:Poore J, Nemecek T (June 2018).
2235:in Earth's surface environment.
1387:calcium and magnesium carbonates
829:Reduction of sulfate to sulfide.
695:
694:
146:
23:
5061:
4995:
4944:
4917:
4892:
4857:
4831:Geochimica et Cosmochimica Acta
4822:
4771:
4708:
4649:
4590:
4555:
4520:
4485:
4450:
4395:
4340:
4283:
4235:10.1016/j.earscirev.2011.02.003
4156:
4033:
3992:
3900:
3866:Geochimica et Cosmochimica Acta
3853:
3814:
3757:
3700:
3661:
3598:
3539:
3463:
3406:
3355:
3260:
3213:
3145:
3132:
3023:
2972:
2929:
2878:
2835:
2809:Geochimica et Cosmochimica Acta
2800:
2473:Brock Biology of Microorganisms
2471:Madigan MT, Martino JM (2006).
2437:Sulfate-reducing microorganisms
2319:
2250:sedimentary exhalative deposits
1882:sulfate-methane transition zone
1870:Dissimilatory sulfate reduction
1495:dissimilatory sulfate reduction
953:Dissimilative sulfate reduction
838:of sulfur compounds (elemental
764:), being a constituent of many
34:needs additional citations for
2757:
2724:
2593:
2583:Turchyn, Alexandra V. (2005).
2489:
2191:mass-independent fractionation
1874:anaerobic oxidation of methane
1840:S are on the order of +21‰.
947:Dissimilative sulfur reduction
867:Assimilative sulfate reduction
810:, and elemental sulfur (S) to
788:into inorganic forms, such as
16:Biogeochemical cycle of sulfur
1:
4576:10.1126/science.282.5393.1459
4514:10.1016/S0012-821X(03)00676-9
4479:10.1016/j.chemgeo.2010.05.012
4373:10.1126/science.281.5381.1342
3847:10.1016/j.chemgeo.2009.10.010
3686:10.1016/j.chemgeo.2023.121533
2829:10.1016/S0016-7037(99)00292-6
2778:10.1126/science.258.5089.1756
2710:10.1016/S0037-0738(00)00176-7
2452:
2284:, temperature and especially
2018:Evolution of the sulfur cycle
1820:which is a ratio in per mill
941:Oxidation in elemental sulfur
915:Oxidation of hydrogen sulfide
5402:Lichens and nitrogen cycling
5257:Marine biogeochemical cycles
4886:10.1016/1352-2310(95)00390-8
4851:10.1016/0016-7037(83)90151-5
4749:10.1126/science.289.5480.756
4189:10.1016/j.margen.2012.12.001
3364:"The microbial sulfur cycle"
3339:10.1007/978-3-540-72682-1_19
2938:Nature Reviews. Microbiology
2666:10.1016/0883-2927(95)00008-8
2518:10.1180/minmag.1994.58A.1.49
2268:will form as by-products of
1788:Although there are 25 known
1391:carbonate-associated sulfate
917:produces elemental sulfur (S
381:Marine biogeochemical cycles
7:
4257:American Journal of Science
4005:American Journal of Science
3331:Microbial Sulfur Metabolism
2844:Journal of Thermal Analysis
2415:
10:
5507:
4549:10.1016/j.epsl.2011.10.030
4296:Earth's Earliest Biosphere
3477:Environmental Microbiology
2735:. New York: Plenum Press.
2260:deposits. Iron sulfides,
2133:. This was likely due to
1931:sulfur isotope systematics
1919:Microbial sulfur oxidation
1666:chemolithotrophic bacteria
1608:Microbial sulfur oxidation
1449:dimethylsulfoniopropionate
1155:Acidifying Emissions (g SO
5440:
5301:
5265:
5141:
4926:Journal of Cereal Science
3886:10.1016/j.gca.2014.11.007
3733:10.1038/s41561-018-0122-8
3368:Frontiers in Microbiology
3226:FEMS Microbiology Reviews
3140:Ehrlich's Geomicrobiology
3086:Frontiers in Microbiology
2733:Sulfate-reducing bacteria
1655:anoxygenic photosynthesis
1323:
1307:
1291:
1275:
1259:
1243:
1227:
1211:
1195:
1179:
1163:
1154:
1151:
997:S: native, or elemental,
5390:Arctic methane emissions
5385:clathrate gun hypothesis
5156:carbonate–silicate cycle
3912:Treatise on Geochemistry
3545:de Vries, Pablo. (2013)
3381:10.3389/fmicb.2011.00241
3099:10.3389/fmicb.2019.00849
2397:and one fourth of all NO
2055:3.5 Ga anoxyogenic
1856:sedimentary settings.
1428:. In the geologic past,
1135:, where it is the major
1077:Sulfur sources and sinks
925:green and purple sulfur
449:Arctic methane emissions
444:clathrate gun hypothesis
359:Carbonate–silicate cycle
5422:Phosphorus assimilation
5324:environmental chemistry
4981:10.1023/A:1026503812267
4866:Atmospheric Environment
4683:10.1073/pnas.1305304110
4619:10.1126/science.1095258
4541:2011E&PSL.312..484G
4506:2004E&PSL.218..331N
4277:10.2475/ajs.299.7-9.697
4124:10.1111/1758-2229.12538
3631:10.1126/science.1205103
3576:Schlesinger WH (1997).
3555:University of Groningen
3497:10.1111/1462-2920.12388
3180:10.1073/pnas.1800367115
2558:10.1126/science.aaq0216
2212:photochemical reactions
2187:Great Oxygenation Event
2087:Great Oxygenation Event
1780:
1766:symbiotic relationships
1369:including: pyrite rich
603:environmental chemistry
126:Sulfur cycle in general
5068:Sulfur as a fertilizer
5044:NASA Earth Observatory
4938:10.1006/jcrs.1998.0241
4294:. In Schopf JW (ed.).
4026:10.2475/ajs.304.10.839
2498:Mineralogical Magazine
2404:As it is an important
2094:Banded iron formations
2039:isotopic fractionation
1909:
1803:Canyon Diablo troilite
1651:purple sulfur bacteria
1634:
1490:
1476:
1159:eq per 100 g protein)
964:Sulfur oxidation state
742:important sulfur cycle
128:
5417:Nitrogen assimilation
5135:Biogeochemical cycles
4215:Earth-Science Reviews
3782:10.1128/mBio.00530-17
3419:Nature Communications
1924:isotope fractionation
1907:
1880:is prevalent. At the
1647:Green sulfur bacteria
1632:
1482:
1470:
1455:cells in the ocean's
806:of hydrogen sulfide,
140:Biogeochemical cycles
124:
5491:Biogeochemical cycle
5432:Planetary boundaries
5336:carbon sequestration
5166:oceanic carbon cycle
4872:(10–11): 1815–1822.
3999:Canfield DE (2004).
3286:10.3390/ijms22126398
2646:Applied Geochemistry
2427:Microbial metabolism
1145:acidifying emissions
873:) in which sulfate (
746:biogeochemical cycle
286:nitrogen and lichens
43:improve this article
5427:Sulfur assimilation
5368:Ocean acidification
5004:Fertilizer Research
4973:2000PlSoi.225...95B
4878:1996AtmEn..30.1815P
4843:1983GeCoA..47..855B
4800:10.1038/nature10511
4792:2011Natur.478..369K
4741:2000Sci...289..756F
4674:2013PNAS..11018407O
4668:(46): 18407–18412.
4611:2004Sci...304.1663P
4605:(5677): 1663–1665.
4570:(5393): 1459–1462.
4471:2010ChGeo.275..221J
4428:10.1038/nature09700
4420:2011Natur.469...80G
4365:1998Sci...281.1342H
4359:(5381): 1342–1346.
4269:1999AmJS..299..697C
4227:2011ESRv..106..161J
4181:2013MarGn...9...51W
4116:2017EnvMR...9..323W
4066:10.1038/nature02974
4058:2004Natur.431..834K
4017:2004AmJS..304..839C
3968:2015AREPS..43..593F
3878:2015GeCoA.149..152P
3839:2010ChGeo.269..364Y
3725:2018NatGe..11..421E
3623:2011Sci...333...74S
3489:2014EnvMi..16.3398W
3431:2010NatCo...1..126P
3171:2018PNAS..115.5786B
3044:2001FrBio..46..431H
2993:1982Natur.296..643J
2950:10.1038/nrmicro1892
2899:1988Natur.333..415K
2821:2000GeCoA..64..233A
2772:(5089): 1756–1757.
2702:2001SedG..140..143M
2690:Sedimentary Geology
2658:1995ApGC...10..373M
2614:1995JGR...100.8893R
2549:2018Sci...360..987P
2510:1994MinM...58...88B
2406:nutrient for plants
2239:Economic importance
1864:Marine sulfur cycle
1148:
1085:ranging from +6 in
1081:Sulfur is found in
871:sulfur assimilation
624:Ocean acidification
434:Atmospheric methane
134:Part of a series on
5016:10.1007/BF00747690
3439:10.1038/ncomms1124
3032:Freshwater Biology
2856:10.1007/BF02547004
1984:seafloor sediments
1910:
1853:disproportionation
1847:could fractionate
1790:isotopes of sulfur
1733:In modern oceans,
1662:Hydrothermal vents
1635:
1600:S. The amount of H
1562:hydrothermal vents
1491:
1477:
1451:(DMSP) from dying
1393:). The amount of
1229:Farmed Crustaceans
1142:
836:Disproportionation
784:Mineralization of
129:
5458:
5457:
5412:Nitrogen fixation
5380:Methane clathrate
5361:mycorrhizal fungi
5314:geochemical cycle
5161:deep carbon cycle
5042:, United States:
4957:Triticum aestivum
4910:978-0-471-92251-3
4786:(7369): 369–373.
4735:(5480): 756–759.
4333:978-0-8137-1198-0
4052:(7010): 834–838.
3713:Nature Geoscience
3549:Riftia pachyptila
3483:(11): 3398–3415.
3348:978-3-540-72679-1
3165:(22): 5786–5791.
2987:(5858): 643–645.
2893:(6172): 415–419.
2731:Barton L (1995).
2622:10.1029/94JD03245
2608:(D5): 8893–8918.
2543:(6392): 987–992.
2482:978-0-13-196893-6
2432:Sulfide intrusion
2422:Sulfur metabolism
2293:sedimentary basin
2197:S value from the
2163:sea level changes
2080:sedimentary rocks
1845:sulfate reduction
1506:organic compounds
1499:organic compounds
1424:which acts as an
1401:input from rivers
1367:sedimentary rocks
1339:
1338:
1113:volcanic activity
758:essential element
738:
737:
593:geochemical cycle
439:Methane clathrate
237:mycorrhizal fungi
227:deep carbon cycle
119:
118:
111:
93:
5498:
5448:
5447:
5331:Biosequestration
5319:chemical cycling
5250:deep water cycle
5220:Phosphorus cycle
5128:
5121:
5114:
5105:
5104:
5071:
5065:
5059:
5058:
5057:
5055:
5034:
5028:
5027:
5010:(1–3): 117–125.
4999:
4993:
4992:
4948:
4942:
4941:
4921:
4915:
4914:
4896:
4890:
4889:
4861:
4855:
4854:
4826:
4820:
4819:
4775:
4769:
4768:
4724:
4715:
4712:
4706:
4705:
4695:
4685:
4653:
4647:
4646:
4594:
4588:
4587:
4559:
4553:
4552:
4535:(3–4): 484–496.
4524:
4518:
4517:
4500:(3–4): 331–345.
4489:
4483:
4482:
4465:(3–4): 221–234.
4459:Chemical Geology
4454:
4448:
4447:
4399:
4393:
4392:
4344:
4338:
4337:
4319:
4300:
4299:
4287:
4281:
4280:
4263:(7–9): 697–723.
4252:
4239:
4238:
4221:(1–2): 161–183.
4210:
4201:
4200:
4160:
4154:
4153:
4143:
4095:
4086:
4085:
4037:
4031:
4030:
4028:
3996:
3990:
3989:
3979:
3947:
3934:
3933:
3904:
3898:
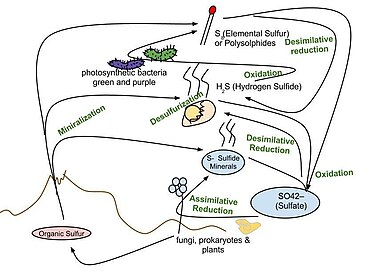
3897:
3857:
3851:
3850:
3833:(3–4): 364–375.
3827:Chemical Geology
3818:
3812:
3811:
3801:
3761:
3755:
3754:
3744:
3704:
3698:
3697:
3674:Chemical Geology
3665:
3659:
3658:
3602:
3596:
3595:
3583:
3573:
3558:
3543:
3537:
3536:
3526:
3508:
3467:
3461:
3460:
3450:
3410:
3404:
3403:
3393:
3383:
3359:
3353:
3352:
3326:
3317:
3316:
3306:
3288:
3264:
3258:
3257:
3217:
3211:
3210:
3200:
3182:
3149:
3143:
3136:
3130:
3129:
3119:
3101:
3077:
3064:
3063:
3027:
3021:
3020:
3001:10.1038/296643a0
2976:
2970:
2969:
2933:
2927:
2926:
2907:10.1038/333415a0
2882:
2876:
2875:
2839:
2833:
2832:
2804:
2798:
2797:
2761:
2755:
2754:
2728:
2722:
2721:
2696:(1–2): 143–175.
2685:
2670:
2669:
2641:
2626:
2625:
2597:
2591:
2590:
2580:
2571:
2570:
2560:
2528:
2522:
2521:
2493:
2487:
2486:
2468:
2391:Washington, D.C.
2270:hydrogen sulfide
2118:Paleoproterozoic
2098:Paleoproterozoic
2092:At 1.8 Ga,
2028:
2005:
2003:
1993:
1991:
1977:
1975:
1956:
1954:
1944:
1942:
1891:sulfate reducers
1792:, only four are
1739:Halothiobacillus
1728:
1727:
1726:
1547:
1546:
1545:
1432:intrusions into
1352:
1351:
1350:
1334:
1333:
1318:
1317:
1302:
1301:
1286:
1285:
1270:
1269:
1254:
1253:
1238:
1237:
1222:
1221:
1206:
1205:
1190:
1189:
1174:
1173:
1149:
1141:
1134:
1133:
1132:
1097:
1096:
1095:
1083:oxidation states
1048:
1047:
1046:
1032:
1031:
1030:
957:sulfate reducers
931:chemolithotrophs
905:hydrogen sulfide
885:) is reduced by
884:
883:
882:
856:hydrogen sulfide
825:
824:
823:
798:sulfide minerals
790:hydrogen sulfide
730:
723:
716:
703:
698:
697:
610:Biosequestration
598:chemical cycling
308:Phosphorus cycle
171:deep water cycle
150:
131:
130:
114:
107:
103:
100:
94:
92:
51:
27:
19:
5506:
5505:
5501:
5500:
5499:
5497:
5496:
5495:
5461:
5460:
5459:
5454:
5436:
5351:biological pump
5309:Biogeochemistry
5297:
5266:Research groups
5261:
5137:
5132:
5080:
5075:
5074:
5066:
5062:
5053:
5051:
5036:
5035:
5031:
5000:
4996:
4967:(1−2): 95–107.
4955:L.) and wheat (
4949:
4945:
4922:
4918:
4911:
4897:
4893:
4862:
4858:
4827:
4823:
4776:
4772:
4725:
4718:
4713:
4709:
4654:
4650:
4595:
4591:
4560:
4556:
4525:
4521:
4490:
4486:
4455:
4451:
4414:(7328): 80–83.
4400:
4396:
4345:
4341:
4334:
4320:
4303:
4288:
4284:
4253:
4242:
4211:
4204:
4169:Marine Genomics
4161:
4157:
4096:
4089:
4038:
4034:
4011:(10): 839–861.
3997:
3993:
3948:
3937:
3930:
3905:
3901:
3858:
3854:
3819:
3815:
3762:
3758:
3705:
3701:
3666:
3662:
3617:(6038): 74–77.
3603:
3599:
3592:
3574:
3561:
3544:
3540:
3468:
3464:
3411:
3407:
3360:
3356:
3349:
3327:
3320:
3265:
3261:
3232:(6): 999–1043.
3218:
3214:
3150:
3146:
3137:
3133:
3078:
3067:
3028:
3024:
2977:
2973:
2934:
2930:
2883:
2879:
2840:
2836:
2805:
2801:
2762:
2758:
2743:
2729:
2725:
2686:
2673:
2642:
2629:
2598:
2594:
2581:
2574:
2529:
2525:
2494:
2490:
2483:
2469:
2460:
2455:
2418:
2400:
2396:
2371:
2364:
2322:
2303:
2258:porphyry copper
2241:
2234:
2225:
2217:
2183:
2148:
2131:ocean chemistry
2111:
2050:Metasedimentary
2026:
2020:
2001:
1999:
1989:
1987:
1973:
1971:
1968:
1952:
1950:
1940:
1938:
1866:
1849:sulfur isotopes
1786:
1774:Vestimentiferan
1756:
1752:
1748:
1725:
1722:
1721:
1720:
1718:
1716:
1712:
1708:
1704:
1700:
1691:
1687:
1683:
1679:
1675:
1610:
1603:
1599:
1595:
1591:
1587:
1551:
1544:
1541:
1540:
1539:
1537:
1535:
1519:
1513:
1489:
1487:
1475:
1465:
1445:Dimethylsulfide
1423:
1357:. However, the
1349:
1346:
1345:
1344:
1342:
1335:
1331:
1330:
1319:
1315:
1314:
1303:
1299:
1298:
1287:
1283:
1282:
1271:
1267:
1266:
1255:
1251:
1250:
1239:
1235:
1234:
1223:
1219:
1218:
1213:Lamb and Mutton
1207:
1203:
1202:
1191:
1187:
1186:
1175:
1171:
1170:
1158:
1137:oxidizing agent
1131:
1128:
1127:
1126:
1124:
1110:
1094:
1091:
1090:
1089:
1087:
1079:
1072:
1065:
1058:
1054:
1045:
1042:
1041:
1040:
1038:
1029:
1026:
1025:
1024:
1022:
1017:
1008:
988:
984:
976:
966:
920:
910:
901:Desulfurization
881:
878:
877:
876:
874:
822:
819:
818:
817:
815:
795:
734:
693:
686:
685:
684:
665:
650:
649:Research groups
642:
641:
640:
619:
588:Biogeochemistry
582:
574:
573:
572:
467:
457:
456:
455:
428:
418:
417:
416:
407:Calcareous ooze
390:Biological pump
385:
375:
365:
364:
363:
343:
333:
332:
331:
260:
250:
249:
248:
189:
179:
178:
177:
160:
127:
115:
104:
98:
95:
52:
50:
40:
28:
17:
12:
11:
5:
5504:
5494:
5493:
5488:
5483:
5481:Soil chemistry
5478:
5473:
5456:
5455:
5453:
5452:
5441:
5438:
5437:
5435:
5434:
5429:
5424:
5419:
5414:
5409:
5404:
5399:
5394:
5393:
5392:
5387:
5377:
5376:
5375:
5365:
5364:
5363:
5358:
5353:
5348:
5343:
5338:
5328:
5327:
5326:
5321:
5316:
5305:
5303:
5302:Related topics
5299:
5298:
5296:
5295:
5290:
5285:
5280:
5275:
5269:
5267:
5263:
5262:
5260:
5259:
5254:
5253:
5252:
5242:
5237:
5232:
5230:Selenium cycle
5227:
5222:
5217:
5216:
5215:
5205:
5203:Nutrient cycle
5200:
5198:Nitrogen cycle
5195:
5190:
5185:
5180:
5178:Hydrogen cycle
5175:
5173:Chlorine cycle
5170:
5169:
5168:
5163:
5158:
5147:
5145:
5139:
5138:
5131:
5130:
5123:
5116:
5108:
5102:
5101:
5096:
5091:
5086:
5079:
5078:External links
5076:
5073:
5072:
5060:
5029:
4994:
4961:Plant and Soil
4953:Brassica napus
4943:
4916:
4909:
4891:
4856:
4837:(5): 855–862.
4821:
4770:
4716:
4707:
4648:
4589:
4554:
4519:
4484:
4449:
4394:
4339:
4332:
4301:
4282:
4240:
4202:
4155:
4110:(4): 323–344.
4087:
4032:
3991:
3962:(1): 593–622.
3935:
3928:
3908:Brimblecombe P
3899:
3852:
3813:
3756:
3719:(6): 421–425.
3699:
3660:
3597:
3590:
3559:
3538:
3462:
3405:
3354:
3347:
3318:
3259:
3212:
3144:
3131:
3065:
3038:(4): 431–451.
3022:
2971:
2944:(6): 441–454.
2928:
2877:
2850:(1): 241–290.
2834:
2815:(2): 233–246.
2799:
2756:
2741:
2723:
2671:
2652:(4): 373–389.
2627:
2592:
2572:
2523:
2488:
2481:
2457:
2456:
2454:
2451:
2450:
2449:
2444:
2439:
2434:
2429:
2424:
2417:
2414:
2398:
2394:
2387:carbon dioxide
2369:
2362:
2321:
2318:
2301:
2240:
2237:
2232:
2223:
2215:
2182:
2179:
2146:
2139:Neoproterozoic
2135:snowball Earth
2109:
2057:photosynthesis
2019:
2016:
1966:
1878:methanogenesis
1865:
1862:
1785:
1779:
1754:
1750:
1746:
1735:Thiomicrospira
1731:
1730:
1723:
1714:
1710:
1706:
1702:
1698:
1694:
1693:
1689:
1685:
1681:
1677:
1673:
1653:(PSB) perform
1619:cable bacteria
1609:
1606:
1601:
1597:
1593:
1589:
1585:
1549:
1542:
1533:
1517:
1511:
1483:
1471:
1464:
1461:
1421:
1409:
1408:
1405:
1402:
1347:
1337:
1336:
1329:
1327:
1321:
1320:
1313:
1311:
1305:
1304:
1297:
1295:
1289:
1288:
1281:
1279:
1273:
1272:
1265:
1263:
1257:
1256:
1249:
1247:
1241:
1240:
1233:
1231:
1225:
1224:
1217:
1215:
1209:
1208:
1201:
1199:
1193:
1192:
1185:
1183:
1177:
1176:
1169:
1167:
1161:
1160:
1156:
1153:
1129:
1108:
1092:
1078:
1075:
1070:
1063:
1056:
1052:
1043:
1027:
1015:
1006:
986:
982:
974:
965:
962:
961:
960:
950:
944:
938:
923:photosynthetic
918:
912:
908:
898:
879:
860:
859:
833:
830:
827:
820:
801:
793:
786:organic sulfur
736:
735:
733:
732:
725:
718:
710:
707:
706:
705:
704:
688:
687:
683:
682:
677:
672:
666:
664:
663:
658:
652:
651:
648:
647:
644:
643:
639:
638:
633:
632:
631:
620:
618:
617:
615:Deep biosphere
612:
607:
606:
605:
600:
595:
584:
583:
581:Related topics
580:
579:
576:
575:
571:
570:
565:
560:
555:
550:
545:
540:
535:
530:
525:
520:
515:
510:
505:
500:
495:
490:
485:
480:
475:
469:
468:
463:
462:
459:
458:
454:
453:
452:
451:
446:
436:
430:
429:
424:
423:
420:
419:
415:
414:
412:Siliceous ooze
409:
404:
403:
402:
397:
395:microbial loop
386:
384:
383:
377:
376:
371:
370:
367:
366:
362:
361:
356:
351:
345:
344:
339:
338:
335:
334:
330:
329:
328:
327:
317:
316:
315:
305:
300:
299:
298:
293:
288:
283:
278:
271:Nitrogen cycle
268:
266:Hydrogen cycle
262:
261:
258:Nutrient cycle
256:
255:
252:
251:
247:
246:
244:Boreal forests
241:
240:
239:
234:
229:
224:
214:
213:
212:
207:
202:
191:
190:
185:
184:
181:
180:
176:
175:
174:
173:
162:
161:
156:
155:
152:
151:
143:
142:
136:
135:
125:
117:
116:
58:"Sulfur cycle"
31:
29:
22:
15:
9:
6:
4:
3:
2:
5503:
5492:
5489:
5487:
5484:
5482:
5479:
5477:
5474:
5472:
5469:
5468:
5466:
5451:
5443:
5442:
5439:
5433:
5430:
5428:
5425:
5423:
5420:
5418:
5415:
5413:
5410:
5408:
5407:Nitrification
5405:
5403:
5400:
5398:
5395:
5391:
5388:
5386:
5383:
5382:
5381:
5378:
5374:
5371:
5370:
5369:
5366:
5362:
5359:
5357:
5354:
5352:
5349:
5347:
5344:
5342:
5339:
5337:
5334:
5333:
5332:
5329:
5325:
5322:
5320:
5317:
5315:
5312:
5311:
5310:
5307:
5306:
5304:
5300:
5294:
5291:
5289:
5286:
5284:
5281:
5279:
5276:
5274:
5271:
5270:
5268:
5264:
5258:
5255:
5251:
5248:
5247:
5246:
5243:
5241:
5238:
5236:
5233:
5231:
5228:
5226:
5223:
5221:
5218:
5214:
5211:
5210:
5209:
5206:
5204:
5201:
5199:
5196:
5194:
5193:Mineral cycle
5191:
5189:
5188:Mercury cycle
5186:
5184:
5181:
5179:
5176:
5174:
5171:
5167:
5164:
5162:
5159:
5157:
5154:
5153:
5152:
5149:
5148:
5146:
5144:
5140:
5136:
5129:
5124:
5122:
5117:
5115:
5110:
5109:
5106:
5100:
5097:
5095:
5092:
5090:
5087:
5085:
5082:
5081:
5069:
5064:
5049:
5046:, acid rain,
5045:
5041:
5040:
5033:
5025:
5021:
5017:
5013:
5009:
5005:
4998:
4990:
4986:
4982:
4978:
4974:
4970:
4966:
4962:
4958:
4954:
4947:
4939:
4935:
4931:
4927:
4920:
4912:
4906:
4902:
4895:
4887:
4883:
4879:
4875:
4871:
4867:
4860:
4852:
4848:
4844:
4840:
4836:
4832:
4825:
4817:
4813:
4809:
4805:
4801:
4797:
4793:
4789:
4785:
4781:
4774:
4766:
4762:
4758:
4754:
4750:
4746:
4742:
4738:
4734:
4730:
4723:
4721:
4711:
4703:
4699:
4694:
4689:
4684:
4679:
4675:
4671:
4667:
4663:
4659:
4652:
4644:
4640:
4636:
4632:
4628:
4624:
4620:
4616:
4612:
4608:
4604:
4600:
4593:
4585:
4581:
4577:
4573:
4569:
4565:
4558:
4550:
4546:
4542:
4538:
4534:
4530:
4523:
4515:
4511:
4507:
4503:
4499:
4495:
4488:
4480:
4476:
4472:
4468:
4464:
4460:
4453:
4445:
4441:
4437:
4433:
4429:
4425:
4421:
4417:
4413:
4409:
4405:
4398:
4390:
4386:
4382:
4378:
4374:
4370:
4366:
4362:
4358:
4354:
4350:
4343:
4335:
4329:
4325:
4318:
4316:
4314:
4312:
4310:
4308:
4306:
4297:
4293:
4286:
4278:
4274:
4270:
4266:
4262:
4258:
4251:
4249:
4247:
4245:
4236:
4232:
4228:
4224:
4220:
4216:
4209:
4207:
4198:
4194:
4190:
4186:
4182:
4178:
4174:
4170:
4166:
4159:
4151:
4147:
4142:
4137:
4133:
4129:
4125:
4121:
4117:
4113:
4109:
4105:
4101:
4094:
4092:
4083:
4079:
4075:
4071:
4067:
4063:
4059:
4055:
4051:
4047:
4043:
4036:
4027:
4022:
4018:
4014:
4010:
4006:
4002:
3995:
3987:
3983:
3978:
3973:
3969:
3965:
3961:
3957:
3953:
3946:
3944:
3942:
3940:
3931:
3929:9780080983004
3925:
3921:
3917:
3913:
3909:
3903:
3895:
3891:
3887:
3883:
3879:
3875:
3871:
3867:
3863:
3856:
3848:
3844:
3840:
3836:
3832:
3828:
3824:
3817:
3809:
3805:
3800:
3795:
3791:
3787:
3783:
3779:
3775:
3771:
3767:
3760:
3752:
3748:
3743:
3738:
3734:
3730:
3726:
3722:
3718:
3714:
3710:
3703:
3695:
3691:
3687:
3683:
3679:
3675:
3671:
3664:
3656:
3652:
3648:
3644:
3640:
3636:
3632:
3628:
3624:
3620:
3616:
3612:
3608:
3601:
3593:
3591:9780126251555
3587:
3582:
3581:
3572:
3570:
3568:
3566:
3564:
3556:
3552:
3550:
3542:
3534:
3530:
3525:
3520:
3516:
3512:
3507:
3502:
3498:
3494:
3490:
3486:
3482:
3478:
3474:
3466:
3458:
3454:
3449:
3444:
3440:
3436:
3432:
3428:
3424:
3420:
3416:
3409:
3401:
3397:
3392:
3387:
3382:
3377:
3373:
3369:
3365:
3358:
3350:
3344:
3340:
3336:
3332:
3325:
3323:
3314:
3310:
3305:
3300:
3296:
3292:
3287:
3282:
3278:
3274:
3270:
3263:
3255:
3251:
3247:
3243:
3239:
3235:
3231:
3227:
3223:
3216:
3208:
3204:
3199:
3194:
3190:
3186:
3181:
3176:
3172:
3168:
3164:
3160:
3156:
3148:
3141:
3135:
3127:
3123:
3118:
3113:
3109:
3105:
3100:
3095:
3091:
3087:
3083:
3076:
3074:
3072:
3070:
3061:
3057:
3053:
3049:
3045:
3041:
3037:
3033:
3026:
3018:
3014:
3010:
3006:
3002:
2998:
2994:
2990:
2986:
2982:
2975:
2967:
2963:
2959:
2955:
2951:
2947:
2943:
2939:
2932:
2924:
2920:
2916:
2912:
2908:
2904:
2900:
2896:
2892:
2888:
2881:
2873:
2869:
2865:
2861:
2857:
2853:
2849:
2845:
2838:
2830:
2826:
2822:
2818:
2814:
2810:
2803:
2795:
2791:
2787:
2783:
2779:
2775:
2771:
2767:
2760:
2752:
2748:
2744:
2742:0-306-44857-2
2738:
2734:
2727:
2719:
2715:
2711:
2707:
2703:
2699:
2695:
2691:
2684:
2682:
2680:
2678:
2676:
2667:
2663:
2659:
2655:
2651:
2647:
2640:
2638:
2636:
2634:
2632:
2623:
2619:
2615:
2611:
2607:
2603:
2596:
2588:
2587:
2579:
2577:
2568:
2564:
2559:
2554:
2550:
2546:
2542:
2538:
2534:
2527:
2519:
2515:
2511:
2507:
2503:
2499:
2492:
2484:
2478:
2474:
2467:
2465:
2463:
2458:
2448:
2445:
2443:
2440:
2438:
2435:
2433:
2430:
2428:
2425:
2423:
2420:
2419:
2413:
2411:
2407:
2402:
2392:
2388:
2383:
2379:
2375:
2374:sulfuric acid
2366:
2360:
2359:Earth's crust
2356:
2350:
2348:
2344:
2340:
2335:
2331:
2327:
2317:
2314:
2309:
2307:
2298:
2294:
2289:
2287:
2283:
2279:
2274:
2271:
2267:
2263:
2259:
2255:
2251:
2246:
2236:
2230:
2221:
2213:
2209:
2204:
2200:
2196:
2192:
2188:
2178:
2175:
2172:
2168:
2164:
2158:
2156:
2152:
2143:
2140:
2136:
2132:
2127:
2124:
2119:
2114:
2107:
2103:
2099:
2095:
2090:
2088:
2083:
2081:
2076:
2072:
2070:
2066:
2062:
2058:
2053:
2051:
2046:
2044:
2040:
2036:
2031:
2023:
2015:
2013:
2007:
1997:
1985:
1981:
1964:
1960:
1948:
1936:
1933:expressed as
1932:
1927:
1925:
1920:
1915:
1906:
1902:
1900:
1896:
1895:methanotrophs
1892:
1887:
1883:
1879:
1875:
1871:
1861:
1857:
1854:
1850:
1846:
1841:
1839:
1834:
1830:
1825:
1823:
1819:
1817:
1812:
1808:
1804:
1800:
1795:
1791:
1783:
1778:
1775:
1771:
1767:
1762:
1760:
1744:
1740:
1736:
1696:
1695:
1688:O + 4 S + 3 H
1671:
1670:
1669:
1667:
1663:
1659:
1656:
1652:
1648:
1644:
1640:
1631:
1627:
1625:
1620:
1615:
1605:
1583:
1578:
1575:
1571:
1565:
1563:
1559:
1553:
1531:
1527:
1523:
1515:
1507:
1502:
1500:
1496:
1486:
1481:
1474:
1469:
1460:
1458:
1454:
1453:phytoplankton
1450:
1446:
1442:
1440:
1435:
1434:coal measures
1431:
1427:
1426:air pollutant
1419:
1415:
1406:
1403:
1400:
1399:
1398:
1396:
1392:
1388:
1384:
1380:
1376:
1372:
1368:
1364:
1360:
1356:
1328:
1326:
1322:
1312:
1310:
1306:
1296:
1294:
1290:
1280:
1278:
1274:
1264:
1262:
1258:
1248:
1246:
1242:
1232:
1230:
1226:
1216:
1214:
1210:
1200:
1198:
1194:
1184:
1182:
1178:
1168:
1166:
1162:
1150:
1146:
1140:
1138:
1122:
1118:
1114:
1106:
1102:
1098:
1084:
1074:
1073:
1066:
1059:
1035:
1034:
1018:
1010:
1009:
1001:
1000:
995:
994:
990:
978:
969:
958:
954:
951:
948:
945:
942:
939:
936:
932:
928:
924:
916:
913:
906:
902:
899:
896:
892:
888:
872:
868:
865:
864:
863:
857:
853:
849:
845:
841:
837:
834:
831:
828:
813:
809:
805:
802:
799:
791:
787:
783:
782:
781:
779:
775:
771:
767:
763:
759:
755:
751:
748:in which the
747:
743:
731:
726:
724:
719:
717:
712:
711:
709:
708:
702:
692:
691:
690:
689:
681:
678:
676:
673:
671:
668:
667:
662:
659:
657:
654:
653:
646:
645:
637:
634:
630:
627:
626:
625:
622:
621:
616:
613:
611:
608:
604:
601:
599:
596:
594:
591:
590:
589:
586:
585:
578:
577:
569:
566:
564:
561:
559:
556:
554:
551:
549:
546:
544:
541:
539:
536:
534:
531:
529:
526:
524:
521:
519:
516:
514:
511:
509:
506:
504:
501:
499:
496:
494:
491:
489:
486:
484:
481:
479:
476:
474:
471:
470:
466:
461:
460:
450:
447:
445:
442:
441:
440:
437:
435:
432:
431:
427:
426:Methane cycle
422:
421:
413:
410:
408:
405:
401:
398:
396:
393:
392:
391:
388:
387:
382:
379:
378:
374:
369:
368:
360:
357:
355:
352:
350:
349:Calcium cycle
347:
346:
342:
337:
336:
326:
323:
322:
321:
318:
314:
311:
310:
309:
306:
304:
301:
297:
294:
292:
289:
287:
284:
282:
281:nitrification
279:
277:
274:
273:
272:
269:
267:
264:
263:
259:
254:
253:
245:
242:
238:
235:
233:
230:
228:
225:
223:
220:
219:
218:
217:Sequestration
215:
211:
208:
206:
203:
201:
198:
197:
196:
193:
192:
188:
183:
182:
172:
169:
168:
167:
164:
163:
159:
154:
153:
149:
145:
144:
141:
138:
137:
133:
132:
123:
113:
110:
102:
91:
88:
84:
81:
77:
74:
70:
67:
63:
60: –
59:
55:
54:Find sources:
48:
44:
38:
37:
32:This article
30:
26:
21:
20:
5476:Soil biology
5240:Sulfur cycle
5239:
5235:Silica cycle
5208:Oxygen cycle
5151:Carbon cycle
5063:
5054:February 15,
5052:, retrieved
5038:
5032:
5007:
5003:
4997:
4964:
4960:
4956:
4952:
4946:
4929:
4925:
4919:
4900:
4894:
4869:
4865:
4859:
4834:
4830:
4824:
4783:
4779:
4773:
4732:
4728:
4710:
4665:
4661:
4651:
4602:
4598:
4592:
4567:
4563:
4557:
4532:
4528:
4522:
4497:
4493:
4487:
4462:
4458:
4452:
4411:
4407:
4397:
4356:
4352:
4342:
4323:
4295:
4285:
4260:
4256:
4218:
4214:
4172:
4168:
4158:
4107:
4103:
4049:
4045:
4035:
4008:
4004:
3994:
3959:
3955:
3911:
3902:
3869:
3865:
3855:
3830:
3826:
3816:
3773:
3769:
3759:
3716:
3712:
3702:
3677:
3673:
3663:
3614:
3610:
3600:
3579:
3554:
3548:
3541:
3480:
3476:
3465:
3422:
3418:
3408:
3371:
3367:
3357:
3330:
3279:(12): 6398.
3276:
3272:
3262:
3229:
3225:
3215:
3162:
3158:
3147:
3139:
3134:
3089:
3085:
3035:
3031:
3025:
2984:
2980:
2974:
2941:
2937:
2931:
2890:
2886:
2880:
2847:
2843:
2837:
2812:
2808:
2802:
2769:
2765:
2759:
2732:
2726:
2693:
2689:
2649:
2645:
2605:
2601:
2595:
2585:
2540:
2536:
2526:
2504:(1): 88–89.
2501:
2497:
2491:
2472:
2403:
2367:
2351:
2334:fossil fuels
2332:, and other
2323:
2320:Human impact
2313:fossil fuels
2310:
2290:
2275:
2245:fossil fuels
2242:
2219:
2202:
2198:
2194:
2184:
2159:
2154:
2150:
2144:
2128:
2115:
2091:
2084:
2077:
2073:
2069:phototrophic
2065:magmatically
2060:
2054:
2047:
2034:
2032:
2024:
2021:
2008:
2004:10 kg/a
1995:
1992:10 kg/a
1979:
1976:10 kg/a
1962:
1958:
1955:10 kg/a
1946:
1934:
1928:
1911:
1867:
1858:
1842:
1837:
1826:
1815:
1810:
1799:Solar System
1787:
1781:
1770:salt marshes
1763:
1742:
1738:
1734:
1732:
1660:
1636:
1611:
1579:
1574:organic-rich
1566:
1558:denaturation
1554:
1525:
1503:
1493:Through the
1492:
1443:
1418:fossil fuels
1410:
1340:
1080:
1036:
1011:
1002:
996:
970:
967:
952:
946:
940:
935:polysulfides
914:
900:
893:and various
866:
861:
778:sulfur cycle
777:
741:
739:
553:ozone–oxygen
465:Other cycles
373:Marine cycle
354:Silica cycle
325:assimilation
320:Sulfur cycle
319:
313:assimilation
303:Oxygen cycle
296:assimilation
276:human impact
187:Carbon cycle
105:
99:January 2021
96:
86:
79:
72:
65:
53:
41:Please help
36:verification
33:
5356:viral shunt
5346:soil carbon
5341:carbon sink
5245:Water cycle
4932:(1): 1–17.
3872:: 152–164.
3742:1874/367905
2330:natural gas
2297:lithologies
2208:ultraviolet
2171:Pleistocene
2123:Proterozoic
2102:iron oxides
1901:complexes.
1886:methanogens
1643:chemotrophs
1639:phototrophs
1624:periplasmic
1536:S (HS) and
1457:photic zone
1261:Farmed Fish
1152:Food Types
1115:as well as
895:prokaryotes
848:thiosulfate
400:viral shunt
232:soil carbon
222:carbon sink
205:terrestrial
200:atmospheric
166:Water cycle
158:Water cycle
5471:Metabolism
5465:Categories
5225:Rock cycle
5183:Iron cycle
3680:: 121533.
3425:(8): 126.
2453:References
2410:acid rains
2355:atmosphere
2347:weathering
2278:evaporites
2266:sphalerite
2071:bacteria.
2027:10 kg
1943:10 kg
1649:(GSB) and
1614:autotrophs
1582:reservoirs
1570:stratified
1522:carbonates
1416:and other
1293:Groundnuts
1117:weathering
869:(see also
341:Rock cycle
69:newspapers
5373:acid rain
5278:GEOTRACES
4816:205226545
4627:0036-8075
4175:: 51–61.
4132:1758-2229
3986:140644882
3894:0016-7037
3790:2161-2129
3751:1752-0908
3694:258600480
3639:0036-8075
3515:1462-2912
3506:1805/9247
3295:1422-0067
3246:1574-6976
3189:0027-8424
3108:1664-302X
3060:0046-5070
3009:0028-0836
2915:0028-0836
2864:0368-4466
2794:129371120
2382:acid rain
2252:(SEDEX),
2126:Archean.
2012:sulfatase
1807:meteorite
1805:(CDT), a
1743:Beggiatoa
1379:anhydrite
1375:evaporite
1359:biosphere
1099:to −2 in
1021:sulfite (
955:in which
929:and some
804:Oxidation
770:cofactors
661:GEOTRACES
629:acid rain
543:manganese
5450:Category
5099:Lenntech
5048:archived
5039:Glossary
5024:42207099
4989:44208638
4808:22012395
4765:12287304
4757:10926533
4702:24170863
4643:10539452
4635:15192227
4436:21209662
4389:13046760
4197:23273849
4150:28419734
4074:15483609
3808:28765215
3647:21719675
3533:24428801
3457:21119639
3400:22144979
3313:34203823
3254:19645821
3207:29735671
3126:31105660
2966:22775967
2958:18461075
2872:95526523
2786:17831655
2751:32311676
2567:29853680
2416:See also
2339:tectonic
2167:Pliocene
1363:seawater
1355:proteins
1101:sulfides
927:bacteria
766:proteins
701:Category
563:vanadium
558:selenium
513:fluorine
503:chromium
498:chlorine
473:aluminum
291:fixation
4969:Bibcode
4874:Bibcode
4839:Bibcode
4788:Bibcode
4737:Bibcode
4729:Science
4693:3831968
4670:Bibcode
4607:Bibcode
4599:Science
4584:9822370
4564:Science
4537:Bibcode
4502:Bibcode
4467:Bibcode
4444:4319979
4416:Bibcode
4381:9721097
4361:Bibcode
4353:Science
4265:Bibcode
4223:Bibcode
4177:Bibcode
4141:5573963
4112:Bibcode
4082:4404486
4054:Bibcode
4013:Bibcode
3964:Bibcode
3874:Bibcode
3835:Bibcode
3799:5539420
3721:Bibcode
3655:1248182
3619:Bibcode
3611:Science
3524:4262008
3485:Bibcode
3448:3060606
3427:Bibcode
3391:3228992
3374:: 241.
3304:8232776
3198:5984516
3167:Bibcode
3117:6492693
3092:: 849.
3040:Bibcode
3017:4308770
2989:Bibcode
2923:4354648
2895:Bibcode
2817:Bibcode
2766:Science
2718:4606551
2698:Bibcode
2654:Bibcode
2610:Bibcode
2545:Bibcode
2537:Science
2506:Bibcode
2368:When SO
2343:erosion
2174:glacial
2165:due to
2043:Archean
2014:genes.
1530:alkanes
1430:igneous
1395:sulfate
1385:), and
1377:rocks (
1245:Poultry
1119:of the
852:sulfate
850:) into
844:sulfite
812:sulfate
808:sulfide
754:geology
548:mercury
538:lithium
493:cadmium
488:bromine
478:arsenic
210:oceanic
83:scholar
5486:Sulfur
5143:Cycles
5022:
4987:
4959:L.)".
4907:
4814:
4806:
4780:Nature
4763:
4755:
4700:
4690:
4641:
4633:
4625:
4582:
4442:
4434:
4408:Nature
4387:
4379:
4330:
4195:
4148:
4138:
4130:
4080:
4072:
4046:Nature
3984:
3926:
3892:
3806:
3796:
3788:
3749:
3692:
3653:
3645:
3637:
3588:
3531:
3521:
3513:
3455:
3445:
3398:
3388:
3345:
3311:
3301:
3293:
3252:
3244:
3205:
3195:
3187:
3124:
3114:
3106:
3058:
3015:
3007:
2981:Nature
2964:
2956:
2921:
2913:
2887:Nature
2870:
2862:
2792:
2784:
2749:
2739:
2716:
2565:
2479:
2447:Sulfur
2306:barite
2264:, and
2262:galena
2229:pyrite
1914:pyrite
1833:barite
1829:gypsum
1794:stable
1759:gypsum
1741:, and
1713:O → CH
1389:(i.e.
1383:baryte
1371:shales
1181:Cheese
1105:pyrite
999:sulfur
907:gas (H
887:plants
840:sulfur
774:sulfur
762:CHNOPS
750:sulfur
699:
523:iodine
508:copper
195:Global
85:
78:
71:
64:
56:
5293:SOLAS
5283:IMBER
5213:ozone
5020:S2CID
4985:S2CID
4812:S2CID
4761:S2CID
4639:S2CID
4440:S2CID
4385:S2CID
4078:S2CID
3982:S2CID
3776:(4).
3690:S2CID
3651:S2CID
3013:S2CID
2962:S2CID
2919:S2CID
2868:S2CID
2790:S2CID
2714:S2CID
2442:Redox
2311:Once
2286:redox
2106:chert
1729:+ 2 H
1705:S + O
1680:S + O
1676:+ 4 H
1341:When
1252:102.4
1236:133.1
1220:139.0
1204:142.7
1188:165.5
1172:343.6
1143:Mean
1121:crust
891:fungi
780:are:
744:is a
680:SOLAS
670:IMBER
483:boron
90:JSTOR
76:books
5288:NOBM
5273:DAAC
5056:2013
4905:ISBN
4804:PMID
4753:PMID
4698:PMID
4631:PMID
4623:ISSN
4580:PMID
4432:PMID
4377:PMID
4328:ISBN
4193:PMID
4146:PMID
4128:ISSN
4070:PMID
3924:ISBN
3890:ISSN
3804:PMID
3786:ISSN
3770:mBio
3747:ISSN
3643:PMID
3635:ISSN
3586:ISBN
3529:PMID
3511:ISSN
3453:PMID
3396:PMID
3343:ISBN
3309:PMID
3291:ISSN
3250:PMID
3242:ISSN
3203:PMID
3185:ISSN
3122:PMID
3104:ISSN
3056:ISSN
3005:ISSN
2954:PMID
2911:ISSN
2860:ISSN
2782:PMID
2747:OCLC
2737:ISBN
2563:PMID
2477:ISBN
2345:and
2326:coal
2185:The
2169:and
2104:and
1899:heme
1893:and
1717:O +
1684:→ CH
1641:and
1516:, CO
1414:coal
1381:and
1325:Tofu
1309:Peas
1300:22.6
1284:53.7
1277:Eggs
1268:65.9
1197:Pork
1165:Beef
1107:(FeS
1062:CaSO
854:and
768:and
740:The
675:NOBM
656:DAAC
568:zinc
533:lead
528:iron
518:gold
62:news
5084:EPA
5012:doi
4977:doi
4965:225
4934:doi
4882:doi
4847:doi
4796:doi
4784:478
4745:doi
4733:289
4688:PMC
4678:doi
4666:110
4615:doi
4603:304
4572:doi
4568:282
4545:doi
4533:312
4510:doi
4498:218
4475:doi
4463:275
4424:doi
4412:469
4369:doi
4357:281
4273:doi
4261:299
4231:doi
4219:106
4185:doi
4136:PMC
4120:doi
4062:doi
4050:431
4021:doi
4009:304
3972:doi
3916:doi
3882:doi
3870:149
3843:doi
3831:269
3794:PMC
3778:doi
3737:hdl
3729:doi
3682:doi
3678:632
3627:doi
3615:333
3519:PMC
3501:hdl
3493:doi
3443:PMC
3435:doi
3386:PMC
3376:doi
3335:doi
3299:PMC
3281:doi
3234:doi
3193:PMC
3175:doi
3163:115
3112:PMC
3094:doi
3048:doi
2997:doi
2985:296
2946:doi
2903:doi
2891:333
2852:doi
2825:doi
2774:doi
2770:258
2706:doi
2694:140
2662:doi
2618:doi
2606:100
2553:doi
2541:360
2514:doi
2502:58A
2000:1.0
1951:1.0
1939:1.3
1889:of
1831:or
1822:(‰)
1753:, H
1749:, O
1709:+ H
1701:+ H
1552:).
1548:(CO
1538:HCO
1365:or
1332:6.7
1316:8.5
1067:);
1037:S:
1012:S:
1005:SCl
1003:S:
993:BaS
981:(CH
971:S:
45:by
5467::
5018:.
5008:43
5006:.
4983:.
4975:.
4963:.
4930:30
4928:.
4880:.
4870:30
4868:.
4845:.
4835:47
4833:.
4810:.
4802:.
4794:.
4782:.
4759:.
4751:.
4743:.
4731:.
4719:^
4696:.
4686:.
4676:.
4664:.
4660:.
4637:.
4629:.
4621:.
4613:.
4601:.
4578:.
4566:.
4543:.
4531:.
4508:.
4496:.
4473:.
4461:.
4438:.
4430:.
4422:.
4410:.
4406:.
4383:.
4375:.
4367:.
4355:.
4351:.
4304:^
4271:.
4259:.
4243:^
4229:.
4217:.
4205:^
4191:.
4183:.
4171:.
4167:.
4144:.
4134:.
4126:.
4118:.
4106:.
4102:.
4090:^
4076:.
4068:.
4060:.
4048:.
4044:.
4019:.
4007:.
4003:.
3980:.
3970:.
3960:43
3958:.
3954:.
3938:^
3922:.
3888:.
3880:.
3868:.
3864:.
3841:.
3829:.
3825:.
3802:.
3792:.
3784:.
3772:.
3768:.
3745:.
3735:.
3727:.
3717:11
3715:.
3711:.
3688:.
3676:.
3672:.
3649:.
3641:.
3633:.
3625:.
3613:.
3609:.
3562:^
3553:"
3527:.
3517:.
3509:.
3499:.
3491:.
3481:16
3479:.
3475:.
3451:.
3441:.
3433:.
3421:.
3417:.
3394:.
3384:.
3370:.
3366:.
3341:.
3321:^
3307:.
3297:.
3289:.
3277:22
3275:.
3271:.
3248:.
3240:.
3230:33
3228:.
3224:.
3201:.
3191:.
3183:.
3173:.
3161:.
3157:.
3120:.
3110:.
3102:.
3090:10
3088:.
3084:.
3068:^
3054:.
3046:.
3036:46
3034:.
3011:.
3003:.
2995:.
2983:.
2960:.
2952:.
2940:.
2917:.
2909:.
2901:.
2889:.
2866:.
2858:.
2848:42
2846:.
2823:.
2813:64
2811:.
2788:.
2780:.
2768:.
2745:.
2712:.
2704:.
2692:.
2674:^
2660:.
2650:10
2648:.
2630:^
2616:.
2604:.
2575:^
2561:.
2551:.
2539:.
2535:.
2512:.
2500:.
2461:^
2378:pH
2328:,
2282:pH
1994:;
1737:,
1719:SO
1697:CO
1672:CO
1645:.
1520:,
1441:.
1373:,
1343:SO
1139:.
1125:SO
1088:SO
1069:SF
1060:,
1055:SO
1039:SO
1023:SO
1019:;
1014:SO
991:;
979:;
889:,
875:SO
846:,
842:,
826:).
816:SO
792:(H
5127:e
5120:t
5113:v
5026:.
5014::
4991:.
4979::
4971::
4940:.
4936::
4913:.
4888:.
4884::
4876::
4853:.
4849::
4841::
4818:.
4798::
4790::
4767:.
4747::
4739::
4704:.
4680::
4672::
4645:.
4617::
4609::
4586:.
4574::
4551:.
4547::
4539::
4516:.
4512::
4504::
4481:.
4477::
4469::
4446:.
4426::
4418::
4391:.
4371::
4363::
4336:.
4279:.
4275::
4267::
4237:.
4233::
4225::
4199:.
4187::
4179::
4173:9
4152:.
4122::
4114::
4108:9
4084:.
4064::
4056::
4029:.
4023::
4015::
3988:.
3974::
3966::
3932:.
3918::
3896:.
3884::
3876::
3849:.
3845::
3837::
3810:.
3780::
3774:8
3753:.
3739::
3731::
3723::
3696:.
3684::
3657:.
3629::
3621::
3594:.
3557:.
3535:.
3503::
3495::
3487::
3459:.
3437::
3429::
3423:1
3402:.
3378::
3372:2
3351:.
3337::
3315:.
3283::
3256:.
3236::
3209:.
3177::
3169::
3128:.
3096::
3062:.
3050::
3042::
3019:.
2999::
2991::
2968:.
2948::
2942:6
2925:.
2905::
2897::
2874:.
2854::
2831:.
2827::
2819::
2796:.
2776::
2753:.
2720:.
2708::
2700::
2668:.
2664::
2656::
2624:.
2620::
2612::
2569:.
2555::
2547::
2520:.
2516::
2508::
2485:.
2399:3
2395:2
2370:2
2363:4
2302:2
2233:2
2224:2
2222:O
2220:p
2216:2
2203:δ
2199:δ
2195:δ
2155:δ
2151:δ
2147:4
2110:2
2061:δ
2035:δ
2002:×
1996:δ
1990:×
1988:4
1986:(
1980:δ
1978:(
1974:×
1972:6
1967:2
1963:δ
1959:δ
1953:×
1947:δ
1941:×
1935:δ
1838:δ
1818:S
1816:δ
1811:δ
1784:S
1782:δ
1755:2
1751:2
1747:2
1724:4
1715:2
1711:2
1707:2
1703:2
1699:2
1692:O
1690:2
1686:2
1682:2
1678:2
1674:2
1602:2
1598:2
1594:2
1590:2
1586:2
1550:2
1543:3
1534:2
1528:-
1526:n
1518:2
1514:S
1512:2
1510:H
1422:2
1348:4
1157:2
1130:4
1109:2
1093:4
1071:6
1064:4
1057:4
1053:2
1051:H
1049:(
1044:4
1033:)
1028:3
1016:2
1007:2
989:S
987:2
985:)
983:3
977:S
975:2
973:H
937:.
919:8
909:2
880:4
858:.
821:4
814:(
800:.
794:2
760:(
729:e
722:t
715:v
112:)
106:(
101:)
97:(
87:·
80:·
73:·
66:·
39:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.