1396:. Fast diffusion to the base metals. Can diffuse to the base metal, resulting in higher remelt temperature, potentially allowing step-brazing with the same alloy. Can erode some base materials or penetrate between grain boundaries of many heat-resistant structural alloys, degrading their mechanical properties. Causes intergranular embrittlement of nickel alloys. Improves wetting of/by some alloys, can be added to Au-Ni-Cr alloy to compensate for wetting loss by chromium addition. In low concentrations improves wetting and lowers melting point of nickel brazes. Rapidly diffuses to base materials, may lower their melting point; especially a concern when brazing thin materials. Diffusion away from the braze increases its remelt temperature; exploited in diffusion brazing.
1811:
metal powder. Flux can also be applied using brazing rods with a coating of flux, or a flux core. In either case, the flux flows into the joint when applied to the heated joint and is displaced by the molten filler metal entering the joint. Excess flux should be removed when the cycle is completed because flux left in the joint can lead to corrosion, impede joint inspection, and prevent further surface finishing operations. Phosphorus-containing brazing alloys can be self-fluxing when joining copper to copper. Fluxes are generally selected based on their performance on particular base metals. To be effective, the flux must be chemically compatible with both the base metal and the filler metal being used. Self-fluxing phosphorus filler alloys produce brittle
1184:
Manganese in some alloys may tend to cause porosity in fillets. Tends to react with graphite molds and jigs. Oxidizes easily, requires flux. Lowers melting point of high-copper brazes. Improves mechanical properties and corrosion resistance of silver-copper-zinc brazes. Cheap, even less expensive than zinc. Part of the Cu-Zn-Mn system is brittle, some ratios can not be used. In some alloys increases mechanical properties and corrosion resistance, by a combination of solid solution strengthening, grain refinement, and segregation on fillet surface and in grain boundaries, where it forms a corrosion-resistant layer. Facilitates wetting of cast iron due to its ability to dissolve carbon. Improves conditions for brazing of carbides.
711:
above 60% yields reduced strength and melts above 900 °C. Silver content above 85% yields reduced strength, high liquidus and high cost. Copper-rich alloys prone to stress cracking by ammonia. Silver-rich brazes (above 67.5% Ag) are hallmarkable and used in jewellery; alloys with lower silver content are used for engineering purposes. Alloys with copper-zinc ratio of about 60:40 contain the same phases as brass and match its color; they are used for joining brass. Small amount of nickel improves strength and corrosion resistance and promotes wetting of carbides. Addition of manganese together with nickel increases fracture toughness. Addition of cadmium yields
1136:
gold-copper brazes. Improves mechanical properties and corrosion resistance of silver-copper-zinc brazes. Nickel content offsets brittleness induced by diffusion of aluminum when brazing aluminum-containing alloys, e.g. aluminum bronzes. In some alloys increases mechanical properties and corrosion resistance, by a combination of solid solution strengthening, grain refinement, and segregation on fillet surface and in grain boundaries, where it forms a corrosion-resistant layer. Extensive intersolubility with iron, chromium, manganese, and others; can severely erode such alloys. Embrittled by zinc, many other low melting point metals, and sulfur.
138:. Since brazing does not melt the base metal of the joint, it allows much tighter control over tolerances and produces a clean joint without the need for secondary finishing. Additionally, dissimilar metals and non-metals (i.e. metalized ceramics) can be brazed. In general, brazing also produces less thermal distortion than welding due to the uniform heating of a brazed piece. Complex and multi-part assemblies can be brazed cost-effectively. Welded joints must sometimes be ground flush, a costly secondary operation that brazing does not require because it produces a clean joint. Another advantage is that the brazing can be coated or
244:
1673:, separation of the liquid from the solid portion; for these the heating through the melting range must be sufficiently fast to avoid this effect. Some alloys show extended plastic range, when only a small portion of the alloy is liquid and most of the material melts at the upper temperature range; these are suitable for bridging large gaps and for forming fillets. Highly fluid alloys are suitable for penetrating deep into narrow gaps and for brazing tight joints with narrow tolerances but are not suitable for filling larger gaps. Alloys with wider melting range are less sensitive to non-uniform clearances.
255:
439:
1701:
757:. Continuous series of solid solutions. Wider melting range than Au-Cu alloys but better corrosion resistance and improved wetting. Frequently alloyed with other metals to reduce proportion of gold while maintaining properties. Copper may be added to lower gold proportion, chromium to compensate for loss of corrosion resistance, and boron for improving wetting impaired by the chromium. Generally no more than 35% Ni is used, as higher Ni/Au ratios have too wide melting range. Low vapor pressure.
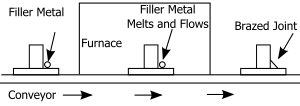
354:. There are many advantages of furnace brazing over other heating methods that make it ideal for mass production. One main advantage is the ease with which it can produce large numbers of small parts that are easily jigged or self-locating. The process also offers the benefits of a controlled heat cycle (allowing use of parts that might distort under localized heating) and no need for post braze cleaning. Common atmospheres used include: inert, reducing or
1684:
strength and increased ductility. Highly accurate melting temperature lets joining process be performed only slightly above the alloy's melting point. On solidifying, there is no mushy state where the alloy appears solid but is not yet; the chance of disturbing the joint by manipulation in such state is reduced (assuming the alloy did not significantly change its properties by dissolving the base metal). Eutectic behavior is especially beneficial for
451:
and the vehicle. Finally, the braze alloy joins the other two materials to create a composite structure, much as layers of wood and glue create plywood. The standard for braze joint strength in many industries is a joint that is stronger than either base material, so that when under stress, one or other of the base materials fails before the joint. Silver brazing may cause defects in certain alloys, e.g. stress-induced inter-granular cracking in
343:
191:
598:(such as stamped washers). Depending on the application, the filler material can be pre-placed at the desired location or applied during the heating cycle. For manual brazing, wire and rod forms are generally used as they are the easiest to apply while heating. In the case of furnace brazing, the alloy is usually placed beforehand since the process is usually highly automated. Some of the more common types of filler metals used are
320:
configuration makes other brazing methods impossible. The main drawback is the high labor cost associated with the method as well as the operator skill required to obtain quality brazed joints. The use of flux or self-fluxing material is required to prevent oxidation. Torch brazing of copper can be done without the use of flux if it is brazed with a torch using oxygen and hydrogen gas, rather than oxygen and other flammable gases.
81:
656:, laminated foils of a carrier metal clad with a layer of braze at each side. The center metal is often copper; its role is to act as a carrier for the alloy, to absorb mechanical stresses due to e.g. differential thermal expansion of dissimilar materials (e.g. a carbide tip and a steel holder), and to act as a diffusion barrier (e.g. to stop diffusion of aluminum from aluminum bronze to steel when brazing these two).
1318:. Can diffuse to the base metal, resulting in higher remelt temperature, potentially allowing step-brazing with the same alloy. At above 0.1% worsens corrosion resistance of nickel alloys. Trace amounts present in stainless steel may facilitate reduction of surface chromium(III) oxide in vacuum and allow fluxless brazing. Diffusion away from the braze increases its remelt temperature; exploited in diffusion brazing.
552:
work piece when heating in a vacuum, greatly reducing residual stresses due to slow heating and cooling cycles. This, in turn, can significantly improve the thermal and mechanical properties of the material, thus providing unique heat treatment capabilities. One such capability is heat-treating or age-hardening the workpiece while performing a metal-joining process, all in a single furnace thermal cycle.
39:
1555:. Most metals, except few (namely silver, copper and gold), form brittle phases with titanium. When brazing ceramics, like other active metals, titanium reacts with them and forms a complex layer on their surface, which in turn is wettable by the silver-copper braze. Wets oxides, carbides, and graphite; frequently a major alloy component for high-temperature brazing of such materials.
519:
and contours are not eroded or changed by the formation of a fillet. Another effect of braze welding is the elimination of stored-up stresses that are often present in fusion welding. This is extremely important in the repair of large castings. The disadvantages are the loss of strength when subjected to high temperatures and the inability to withstand high stresses.
146:
damaged under high service temperatures. Brazed joints require a high degree of base-metal cleanliness when done in an industrial setting. Some brazing applications require the use of adequate fluxing agents to control cleanliness. The joint color is often different from that of the base metal, creating an aesthetic disadvantage.
781:
lower-melting point metals, e.g. zinc. Boron, phosphorus, silicon and carbon lower melting point and rapidly diffuse to base metals. This allows diffusion brazing, and lets the joint be used above the brazing temperature. Borides and phosphides form brittle phases. Amorphous preforms can be made by rapid solidification.
177:
extent to which these effects are present. In general, however, most production processes are selected to minimize brazing time and associated costs. This is not always the case, however, since in some non-production settings, time and cost are secondary to other joint attributes (e.g., strength, appearance).
747:
corrosion resistance. To maintain corrosion resistance, gold must be kept above 60%. High-temperature strength and corrosion resistance can be improved by further alloying, e.g., with chromium, palladium, manganese, and molybdenum. Added vanadium allows wetting ceramics. Gold-copper has low vapor pressure.
1135:
Strong, corrosion-resistant. Impedes flow of the melt. Addition to gold-copper alloys improves ductility and resistance to creep at high temperatures. Addition to silver allows wetting of silver-tungsten alloys and improves bond strength. Improves wetting of copper-based brazes. Improves ductility of
542:
being used although true welding with cast iron rods is also available. Ductile cast iron pipe may be also "cadwelded," a process that connects joints by means of a small copper wire fused into the iron when previously ground down to the bare metal, parallel to the iron joints being formed as per hub
518:
Braze welding has many advantages over fusion welding. It allows the joining of dissimilar metals, minimization of heat distortion, and can reduce the need for extensive pre-heating. Additionally, since the metals joined are not melted in the process, the components retain their original shape; edges
333:
is a method that almost eliminates the need for manual labor in the brazing operation, except for loading and unloading of the machine. The main advantages of this method are: a high production rate, uniform braze quality, and reduced operating cost. The equipment used is essentially the same as that
308:
brazing is by far the most common method of mechanized brazing in use. It is best used in small production volumes or in specialized operations, and in some countries, it accounts for a majority of the brazing taking place. There are three main categories of torch brazing in use: manual, machine, and
1691:
Metals with fine grain structure before melting provide superior wetting to metals with large grains. Alloying additives (e.g. strontium to aluminum) can be added to refine grain structure, and the preforms or foils can be prepared by rapid quenching. Very rapid quenching may provide amorphous metal
1652:
Some additives and impurities act at very low levels. Both positive and negative effects can be observed. Strontium at levels of 0.01% refines grain structure of aluminum. Beryllium and bismuth at similar levels help disrupt the passivation layer of aluminum oxide and promote wetting. Carbon at 0.1%
746:
Gold-copper. Continuous series of solid solutions. Readily wet many metals, including refractory ones. Narrow melting ranges, good fluidity. Frequently used in jewellery. Alloys with 40–90% of gold harden on cooling but stay ductile. Nickel improves ductility. Silver lowers melting point but worsens
703:
Copper-zinc. General purpose, used for joining steel and cast iron. Corrosion resistance usually inadequate for copper, silicon bronze, copper-nickel, and stainless steel. Reasonably ductile. High vapor pressure due to volatile zinc, unsuitable for furnace brazing. Copper-rich alloys prone to stress
373:
type furnace has relatively low initial equipment costs, and can heat each part load separately. It can be turned on and off at will, which reduces operating expenses when it's not in use. These furnaces are suited to medium to large volume production, and offer a large degree of flexibility in type
326:
is commonly used where a repetitive braze operation is being carried out. This method is a mix of both automated and manual operations with an operator often placing brazes material, flux and jigging parts while the machine mechanism carries out the actual braze. The advantage of this method is that
1810:
is required to prevent oxides from forming while the metal is heated. The flux also serves the purpose of cleaning any contamination left on the brazing surfaces. Flux can be applied in any number of forms including flux paste, liquid, powder or pre-made brazing pastes that combine flux with filler
1708:
For successful wetting, the base metal must be at least partially soluble in at least one component of the brazing alloy. The molten alloy therefore tends to attack the base metal and dissolve it, slightly changing its composition in the process. The composition change is reflected in the change of
590:
A variety of alloys are used as filler metals for brazing depending on the intended use or application method. In general, braze alloys are composed of three or more metals to form an alloy with the desired properties. The filler metal for a particular application is chosen based on its ability to:
319:
placed on or near the joint being brazed. The torch can either be hand held or held in a fixed position depending on whether the operation is completely manual or has some level of automation. Manual brazing is most commonly used on small production volumes or in applications where the part size or
262:
There are many heating methods available to accomplish brazing operations. The most important factor in choosing a heating method is achieving efficient transfer of heat throughout the joint and doing so within the heat capacity of the individual base metals used. The geometry of the braze joint is
158:
Another consideration is the effect of temperature and time on the quality of brazed joints. As the temperature of the braze alloy is increased, the alloying and wetting action of the filler metal increases as well. In general, the brazing temperature selected must be above the melting point of the
1508:
can be brazed with phosphorus bearing alloy to produce joints that shatter easily at detonation.) Avoid in environments with presence of sulfur dioxide (e.g. paper mills) and hydrogen sulfide (e.g. sewers, or close to volcanoes); the phosphorus-rich phase rapidly corrodes in presence of sulfur and
1183:
High vapor pressure, unsuitable for vacuum brazing. In gold-based alloys increases ductility. Increases corrosion resistance of copper and nickel alloys. Improves high-temperature strength and corrosion resistance of gold-copper alloys. Higher manganese content may aggravate tendency to liquation.
1158:
Corrosion-resistant. Increases high-temperature corrosion resistance and strength of gold-based alloys. Added to copper and nickel to increase corrosion resistance of them and their alloys. Wets oxides, carbides, and graphite; frequently a major alloy component for high-temperature brazing of such
710:
Silver-copper-zinc. Lower melting point than Ag-Cu for same Ag content. Combines advantages of Ag-Cu and Cu-Zn. At above 40% Zn the ductility and strength drop, so only lower-zinc alloys of this type are used. At above 25% zinc less ductile copper-zinc and silver-zinc phases appear. Copper content
551:
Vacuum brazing is a material joining technique that offers significant advantages: extremely clean, superior, flux-free braze joints of high integrity and strength. The process can be expensive because it must be performed inside a vacuum chamber vessel. Temperature uniformity is maintained on the
450:
thick. The braze alloy joins the materials and compensates for the difference in their expansion rates. It also provides a cushion between the hard carbide tip and the hard steel, which softens impact and prevents tip loss and damage—much as a vehicle's suspension helps prevent damage to the tires
410:
materials and other exotic alloy combinations unsuited to atmosphere furnaces. Due to the absence of flux or a reducing atmosphere, the part cleanliness is critical when brazing in a vacuum. The three main types of vacuum furnace are: single-wall hot retort, double-walled hot retort, and cold-wall
387:
furnaces differ from other batch-type furnaces in that they make use of a sealed lining called a "retort". The retort is generally sealed with either a gasket or is welded shut and filled completely with the desired atmosphere and then heated externally by conventional heating elements. Due to the
358:
atmospheres all of which protect the part from oxidation. Some other advantages include: low unit cost when used in mass production, close temperature control, and the ability to braze multiple joints at once. Furnaces are typically heated using either electric, gas or oil depending on the type of
1014:
Lowers melting point, improves fluidity. Toxic. Produces toxic fumes, requires ventilation. High affinity to oxygen, promotes wetting of copper in air by reduction of the cuprous oxide surface film. Less such benefit in furnace brazing with controlled atmosphere. Allows reducing silver content of
990:
Excellent corrosion resistance, though less than gold. Higher mechanical strength than gold. Good high-temperature strength. Very expensive, though less than gold. Makes the joint less prone to fail due to intergranular penetration when brazing alloys of nickel, molybdenum, or tungsten. Increases
921:
in some environments, which may cause joint failure. Traces of bismuth and beryllium together with tin or zinc in aluminum-based braze destabilize oxide film on aluminum, facilitating its wetting. High affinity to oxygen, promotes wetting of copper in air by reduction of the cuprous oxide surface
145:
One of the main disadvantages is the lack of joint strength as compared to a welded joint due to the softer filler metals used. The strength of the brazed joint is likely to be less than that of the base metal(s) but greater than the filler metal. Another disadvantage is that brazed joints can be
1765:
A sacrificial layer of a noble metal can be used on the base metal as an oxygen barrier, preventing formation of oxides and facilitating fluxless brazing. During brazing, the noble metal layer dissolves in the filler metal. Copper or nickel plating of stainless steels performs the same function.
1721:
Wetting of base metals can be improved by adding a suitable metal to the alloy. Tin facilitates wetting of iron, nickel, and many other alloys. Copper wets ferrous metals that silver does not attack, copper-silver alloys can therefore braze steels silver alone won't wet. Zinc improves wetting of
916:
Lowers melting point. Often used with copper. Susceptible to corrosion. Improves wetting on ferrous metals and on nickel alloys. Compatible with aluminum. High vapor tension, produces somewhat toxic fumes, requires ventilation; highly volatile above 500 °C. At high temperatures may boil and
1683:
Eutectic alloys melt at single temperature, without mushy region. Eutectic alloys have superior spreading; non-eutectics in the mushy region have high viscosity and at the same time attack the base metal, with correspondingly lower spreading force. Fine grain size gives eutectics both increased
380:
furnaces are best suited to a steady flow of similar-sized parts through the furnace. These furnaces are often conveyor fed, moving parts through the hot zone at a controlled speed. It is common to use either controlled atmosphere or pre-applied flux in continuous furnaces. In particular, these
176:
In some cases, a worker may select a higher temperature to accommodate other factors in the design (e.g., to allow use of a different filler metal, or to control metallurgical effects, or to sufficiently remove surface contamination). The effect of time on the brazed joint primarily affects the
991:
high-temperature strength of gold-based alloys. Improves high-temperature strength and corrosion resistance of gold-copper alloys. Forms solid solutions with most engineering metals, does not form brittle intermetallics. High oxidation resistance at high temperatures, especially Pd-Ni alloys.
868:
Enhances capillary flow, improves corrosion resistance of less-noble alloys, worsens corrosion resistance of gold and palladium. Relatively expensive. High vapor pressure, problematic in vacuum brazing. Wets copper. Does not wet nickel and iron. Reduces melting point of many alloys, including
154:
and joint strength; in some brazing operations, however, it is not uncommon to have joint clearances around 0.6 mm (0.024 in). Cleanliness of the brazing surfaces is also important, as any contamination can cause poor wetting (flow). The two main methods for cleaning parts, prior to
1749:
The potentially detrimental phases may be distributed evenly through the volume of the alloy, or be concentrated on the braze-base interface. A thick layer of interfacial intermetallics is usually considered detrimental due to its commonly low fracture toughness and other sub-par mechanical
1206:
Increases high-temperature corrosion and strength of gold-based alloys. Increases ductility of gold-based alloys, promotes their wetting of refractory materials, namely carbides and graphite. When present in alloys being joined, may destabilize the surface oxide layer (by oxidizing and then
2050:
A brazing preform is a high quality, precision metal stamping used for a variety of joining applications in manufacturing electronic devices and systems. Typical brazing preform uses include attaching electronic circuitry, packaging electronic devices, providing good thermal and electrical
1753:
On wetting, brazes may liberate elements from the base metal. For example, aluminum-silicon braze wets silicon nitride, dissociates the surface so it can react with silicon, and liberates nitrogen, which may create voids along the joint interface and lower its strength. Titanium-containing
780:
Nickel alloys, even more numerous than silver alloys. High strength. Lower cost than silver alloys. Good high-temperature performance, good corrosion resistance in moderately aggressive environments. Often used for stainless steels and heat-resistant alloys. Embrittled with sulfur and some
922:
film. Less such benefit in furnace brazing with controlled atmosphere. Embrittles nickel. High levels of zinc may result in a brittle alloy. Prone to interfacial corrosion in contact with stainless steel in wet and humid environments. Unsuitable for furnace brazing due to volatility.
1509:
the joint fails. Phosphorus can be also present as an impurity introduced from e.g. electroplating baths. In low concentrations improves wetting and lowers melting point of nickel brazes. Diffusion away from the braze increases its remelt temperature; exploited in diffusion brazing.
435:" (carbide, ceramics, cermet, and similar) tips to tools such as saw blades. "Pretinning" is often done: the braze alloy is melted onto the hard metal tip, which is placed next to the steel and remelted. Pretinning gets around the problem that hard metals are difficult to wet.
1733:
of iron and nickel, phosphorus-containing alloys are therefore unsuitable for brazing nickel and ferrous alloys. Boron tends to diffuse into the base metals, especially along the grain boundaries, and may form brittle borides. Carbon can negatively influence some steels.
1761:
Metals may diffuse from one base alloy to the other one, causing embrittlement or corrosion. An example is diffusion of aluminum from aluminum bronze to a ferrous alloy when joining these. A diffusion barrier, e.g. a copper layer (e.g. in a trimet strip), can be used.
155:
brazing, are chemical cleaning and abrasive or mechanical cleaning. In the case of mechanical cleaning it is important to maintain the proper surface roughness, as wetting on a rough surface occurs much more readily than on a smooth surface of the same geometry.
1932:. Dissociated ammonia (75% hydrogen, 25% nitrogen) can be used for many types of brazing and annealing. Inexpensive. For copper, silver, nickel, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, nickel alloys, Monel, medium and high
773:
Palladium. Good high-temperature performance, high corrosion resistance (less than gold), high strength (more than gold). usually alloyed with nickel, copper, or silver. Forms solid solutions with most metals, does not form brittle intermetallics. Low vapor
1503:
P) and iron, phosphorus alloys unsuitable for brazing alloys bearing iron, nickel or cobalt in amount above 3%. The phosphides segregate at grain boundaries and cause intergranular embrittlement. (Sometimes the brittle joint is actually desired, though.
2018:, AWS type 9. Non-oxidizing, more expensive than nitrogen. Inert. Parts must be very clean, gas must be pure. For copper, silver, nickel, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, nickel alloys, Monel, medium and high
575:
form. Then the assemblies are dipped into a bath of molten salt (typically NaCl, KCl and other compounds), which functions as both heat transfer medium and flux. Many dip brazed parts are used in heat transfer applications for the aerospace industry.
558:
Vacuum brazing is often conducted in a furnace; this means that several joints can be made at once because the whole workpiece reaches the brazing temperature. The heat is transferred using radiation, as many other methods cannot be used in a vacuum.
1781:
is not sensitive to this effect, however the most readily available grades, e.g. electrolytic copper or high-conductivity copper, are. The embrittled joint may then fail catastrophically without any previous sign of deformation or deterioration.
1344:. Improves wetting of copper-based brazes. Promotes flow. Causes intergranular embrittlement of nickel alloys. Rapidly diffuses into the base metals. Diffusion away from the braze increases its remelt temperature; exploited in diffusion brazing.
1777:. The hydrogen present in the flame or atmosphere at high temperature reacts with the oxide, yielding metallic copper and water vapour, steam. The steam bubbles exert high pressure in the metal structure, leading to cracks and joint porosity.
767:. Improved corrosion resistance over Au-Cu and Au-Ni alloys. Used for joining superalloys and refractory metals for high-temperature applications, e.g. jet engines. Expensive. May be substituted for by cobalt-based brazes. Low vapor pressure.
1722:
ferrous metals, indium as well. Aluminum improves wetting of aluminum alloys. For wetting of ceramics, reactive metals capable of forming chemical compounds with the ceramic (e.g. titanium, vanadium, zirconium...) can be added to the braze.
149:
High-quality brazed joints require that parts be closely fitted with base metal surfaces exceptionally clean and free of oxides. In most cases, joint clearances of 0.03 to 0.08 mm (0.0012 to 0.0031 in) are recommended for the best
1741:
between the braze and the base metal, and especially between dissimilar base metals being brazed together. Formation of brittle intermetallic compounds on the alloy interface can cause joint failure. This is discussed more in-depth with
1823:
As brazing work requires high temperatures, oxidation of the metal surface occurs in an oxygen-containing atmosphere. This may necessitate the use of an atmospheric environment other than air. The commonly used atmospheres are:
1709:
the alloy's melting point and the corresponding change of fluidity. For example, some alloys dissolve both silver and copper; dissolved silver lowers their melting point and increases fluidity, copper has the opposite effect.
2036:
Requires evacuating the work chamber. Expensive. Unsuitable (or requires special care) for metals with high vapor pressure, e.g. silver, zinc, phosphorus, cadmium, and manganese. Used for highest-quality joints, for e.g.
1069:. Traces of bismuth and beryllium together with tin or zinc in aluminum-based braze destabilize oxide film on aluminum, facilitating its wetting. Low solubility in zinc, which limits its content in zinc-bearing alloys.
427:
Silver brazing, sometimes known as hard soldering, is brazing using a silver alloy based filler. These silver alloys consist of many different percentages of silver and other metals, such as copper, zinc and cadmium.
327:
it reduces the high labor and skill requirement of manual brazing. The use of flux is also required for this method as there is no protective atmosphere, and it is best suited to small to medium production volumes.
1725:
Dissolution of base metals can cause detrimental changes in the brazing alloy. For example, aluminum dissolved from aluminum bronzes can embrittle the braze; addition of nickel to the braze can offset this.
263:
also a crucial factor to consider, as is the rate and volume of production required. The easiest way to categorize brazing methods is to group them by heating method. Here are some of the most common:
1495:
Lowers melting point. Deoxidizer, decomposes copper oxide; phosphorus-bearing alloys can be used on copper without flux. Does not decompose zinc oxide, so flux is needed for brass. Forms brittle
1090:
Lowers melting point. May disrupt surface oxides. Traces of bismuth and beryllium together with tin or zinc in aluminum-based braze destabilize oxide film on aluminum, facilitating its wetting.
2246:
AWS A3.0:2001, Standard
Welding Terms and Definitions Including Terms for Adhesive Bonding, Brazing, Soldering, Thermal Cutting, and Thermal Spraying, American Welding Society (2001), p. 118.
1667:
Alloys with larger span of solidus/liquidus temperatures tend to melt through a "mushy" state, during which the alloy is a mixture of solid and liquid material. Some alloys show tendency to
725:. Widely used for copper and copper alloys. Does not require flux for copper. Can be also used with silver, tungsten, and molybdenum. Copper-rich alloys prone to stress cracking by ammonia.
1712:
The melting point change can be exploited. As the remelt temperature can be increased by enriching the alloy with dissolved base metal, step brazing using the same braze can be possible.
891:
Good mechanical properties. Often used with silver. Dissolves and wets nickel. Somewhat dissolves and wets iron. Copper-rich alloys sensitive to stress cracking in presence of ammonia.
2051:
conductivity, and providing an interface for electronic connections. Square, rectangular and disc shaped brazing preforms are commonly used to attach electronic components containing
1729:
The effect works both ways; there can be detrimental interactions between the braze alloy and the base metal. Presence of phosphorus in the braze alloy leads to formation of brittle
388:
high temperatures involved, the retort is usually made of heat resistant alloys that resist oxidation. Retort furnaces are often either used in a batch or semi-continuous versions.
142:
for protective purposes. Finally, brazing is easily adapted to mass production and it is easy to automate because the individual process parameters are less sensitive to variation.
1263:
Addition to aluminum makes the alloy suitable for vacuum brazing. Volatile, though less than zinc. Vaporization promotes wetting by removing oxides from the surface, vapors act as
359:
furnace and application. However, some of the disadvantages of this method include: high capital equipment cost, more difficult design considerations and high power consumption.
571:
because air is excluded, thus preventing the formation of oxides. The parts to be joined are fixtured and the brazing compound applied to the mating surfaces, typically in
110:
through the use of a higher temperature and much more closely fitted parts. During the brazing process, the filler metal flows into the gap between close-fitting parts by
2059:. Rectangular frame shaped preforms are often required for the construction of electronic packages while washer shaped brazing preforms are typically utilized to attach
790:
alloys. Good high-temperature corrosion resistance, possible alternative to Au-Pd brazes. Low workability at low temperatures, preforms prepared by rapid solidification.
503:
workpieces. The equipment needed for braze welding is basically identical to the equipment used in brazing. Since braze welding usually requires more heat than brazing,
480:
installations. The method uses a silver- and flux-containing brazing pin, which is melted in the eye of a cable lug. The equipment is normally powered from batteries.
1438:
in trace quantities, improves fluidity of brazes. Particularly useful for alloys of four or more components, where the other additives compromise flow and spreading.
126:) and is then cooled to join the work pieces together. A major advantage of brazing is the ability to join the same or different metals with considerable strength.
1530:
Deoxidizer. Eliminates the need for flux with some materials. Lithium oxide formed by reaction with the surface oxides is easily displaced by molten braze alloy.
2589:
543:
pipe with neoprene gasket seals. The purpose behind this operation is to use electricity along the copper for keeping underground pipes warm in cold climates.
1996:
to some alloys. For copper, silver, nickel, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, nickel alloys, Monel, medium and high
60:
2088:
731:
Like Cu-P, with improved flow. Better for larger gaps. More ductile, better electrical conductivity. Copper-rich alloys prone to stress cracking by ammonia.
2618:
591:
wet the base metals, withstand the service conditions required, and melt at a lower temperature than the base metals or at a very specific temperature.
3009:
1964:, 1–10% CO. For copper, silver, nickel, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, low-nickel alloys, medium and high
1750:
properties. In some situations, e.g. die attaching, it however does not matter much as silicon chips are not typically subjected to mechanical abuse.
2669:
1837:. Acid cleaning bath or mechanical cleaning can be used to remove the oxidation after work. Flux counteracts the oxidation, but may weaken the joint.
1980:. For copper, silver, nickel, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, low-nickel alloys, Monel, medium and high
2006:
Various volatile fluorides, AWS type 8. Special purpose. Can be mixed with atmospheres AWS 1–5 to replace flux. Used for silver-brazing of brasses.
1656:
In some cases, especially for vacuum brazing, high-purity metals and alloys are used. 99.99% and 99.999% purity levels are available commercially.
297:
These heating methods are classified through localised and diffuse heating techniques and offer advantages based on their different applications.
2166:
679:. Good melting properties. Silver enhances flow. Eutectic alloy used for furnace brazing. Copper-rich alloys prone to stress cracking by ammonia.
1754:
nickel-gold braze wets silicon nitride and reacts with its surface, forming titanium nitride and liberating silicon; silicon then forms brittle
1112:
Traces of bismuth and beryllium together with tin or zinc in aluminum-based braze destabilize oxide film on aluminum, facilitating its wetting.
350:
Furnace brazing is a semi-automatic process used widely in industrial brazing operations due to its adaptability to mass production and use of
1976:
Cryogenic or purified (AWS type 6C). Non-oxidizing, economical. At high temperatures can react with some metals, e.g. certain steels, forming
2971:
419:) to 0.00013 Pa (10 Torr) or lower. Vacuum furnaces are most commonly batch-type, and they are suited to medium and high production volumes.
1638:
Compromises integrity of nickel alloys. Can enter the joints from residues of lubricants, grease or paint. Forms brittle nickel sulfide (Ni
715:
alloys with improved fluidity and wetting and lower melting point; however cadmium is toxic. Addition of tin can play mostly the same role.
1769:
In brazing copper, a reducing atmosphere (or even a reducing flame) may react with the oxygen residues in the metal, which are present as
1289:
Lowers melting point. Improves wetting of ferrous alloys by copper-silver alloys. Suitable for joining parts that will be later coated by
159:
filler metal. However, several factors influence the joint designer's temperature selection. The best temperature is usually selected to:
2759:
2572:
2472:
1659:
Care must be taken to not introduce deleterious impurities from joint contamination or by dissolution of the base metals during brazing.
2538:
555:
Products that are most commonly vacuum-brazed include aluminum cold plates, plate-fin heat exchangers, and flat tube heat exchangers.
47:
2275:
1916:. For copper, silver, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, low-nickel alloys, medium and high
2208:
2693:
1815:
if used on iron or nickel. As a general rule, longer brazing cycles should use less active fluxes than short brazing operations.
334:
used for
Machine torch brazing, with the main difference being that the machinery replaces the operator in the part preparation.
2810:
2342:
3002:
1876:. For copper, silver, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, nickel alloys, Monel, medium
1015:
Ag-Cu-Zn alloys. Replaced by tin in more modern alloys. In EU since
December 2011 allowed only for aerospace and military use.
2725:
381:
furnaces offer the benefit of very low manual labor requirements and so are best suited to large scale production operations.
374:
of parts that can be brazed. Either controlled atmospheres or flux can be used to control oxidation and cleanliness of parts.
2928:
2909:
2793:"CDC – NIOSH Publications and Products – Criteria for a Recommended Standard: Welding, Brazing, and Thermal Cutting (88-110)"
2566:
2532:
2466:
1992:
AWS type 7. Strong deoxidizer, highly thermally conductive. Can be used for copper brazing and annealing steel. May cause
2417:
394:
is a relatively economical method of oxide prevention and is most often used to braze materials with very stable oxides (
1576:
Wets oxides, carbides, and graphite; frequently a major alloy component for high-temperature brazing of such materials.
1758:
and eutectic gold-silicon phase; the resulting joint is weak and melts at much lower temperature than may be expected.
2995:
2964:
2844:
2753:
2251:
1894:. For copper, silver, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, low-nickel alloys,
230:
2775:
1229:
Good high-temperature properties and corrosion resistance. In nuclear applications can absorb neutrons and build up
1718:
Nonhomogenous microstructure of the braze may cause non-uniform melting and localized erosions of the base metal.
526:
and ceramic tips are plated and then joined to steel to make tipped band saws. The plating acts as a braze alloy.
2944:
1653:
impairs corrosion resistance of nickel alloys. Aluminum can embrittle mild steel at 0.001%, phosphorus at 0.01%.
3317:
2614:
508:
212:
1057:
Lowers melting point, improves fluidity. Broadens melting range. Can be used with copper, with which it forms
2856:
Kent White, "Authentic
Aluminum Gas Welding: Plus Brazing & Soldering." Publisher: TM Technologies, 2008.
2118:
659:
Brazing alloys form several distinct groups; the alloys in the same group have similar properties and uses.
3023:
2957:
2665:
2096:
1551:
Most commonly used active metal. Few percents added to Ag-Cu alloys facilitate wetting of ceramics, e.g.
689:. Similar to Cu-Zn, used in jewelry due to its high silver content so that the product is compliant with
2163:
1417:
in amount of about 0.08%, can be used to substitute boron where boron would have detrimental effects.
2593:
1715:
Alloys that do not significantly attack the base metals are more suitable for brazing thin sections.
1159:
materials. Impairs wetting by gold-nickel alloys, which can be compensated for by addition of boron.
809:
Containing active metals, e.g., titanium or vanadium. Used for brazing non-metallic materials, e.g.
3239:
3080:
208:
243:
3291:
3110:
3085:
3075:
1392:. Unsuitable for nuclear reactors, as boron is a potent neutron absorber and therefore acts as a
201:
139:
52:
3135:
3130:
3070:
3065:
3019:
2063:
and hermetic feed-throughs to electronic circuits and packages. Some preforms are also used in
1993:
1774:
645:
585:
2743:
2556:
2456:
2522:
2056:
1505:
693:. The color matches silver, and it is resistant to ammonia-containing silver-cleaning fluids.
99:
into the joint, with the filler metal having a lower melting point than the adjoining metal.
2639:
1855:. For silver, copper-phosphorus and copper-zinc filler metals. For brazing copper and brass.
438:
3120:
2872:
2092:
254:
248:
362:
There are four main types of furnaces used in brazing operations: batch type; continuous;
8:
1795:
477:
406:) that cannot be brazed in atmosphere furnaces. Vacuum brazing is also used heavily with
2876:
2216:
515:) fuel is commonly used. The name comes from the fact that no capillary action is used.
2888:
2366:
2060:
1778:
1738:
1700:
918:
2741:
1366:
Lowers melting point. Expensive. For special applications. May create brittle phases.
95:-joining process in which two or more metal items are joined by melting and flowing a
3266:
3261:
3196:
3090:
3039:
2924:
2905:
2892:
2840:
2792:
2749:
2562:
2528:
2462:
2247:
1803:
273:
119:
2880:
2800:
2722:
2663:
1478:
1290:
1066:
432:
151:
111:
2853:
P.M. Roberts, "Industrial
Brazing Practice", CRC Press, Boca Raton, Florida, 2004.
594:
Braze alloy is generally available as rod, ribbon, powder, paste, cream, wire and
3171:
3115:
2860:
2779:
2729:
2170:
2084:
1755:
1552:
1234:
1062:
476:. It has been developed especially for connecting cables to railway track or for
351:
19:
This article is about the metal-joining process. For the cooking technique, see
3219:
3140:
2343:"Vacuum Brazing of Aluminum Cold Plates and Heat Exchangers – Lytron Inc"
2072:
1677:
1486:
1393:
2421:
1950:. For copper, silver, nickel, copper-phosphorus and copper-zinc filler metals.
1833:
Simple and economical. Many materials susceptible to oxidation and buildup of
411:
retort. Typical vacuum levels for brazing range from pressures of 1.3 to 0.13
3311:
3214:
3209:
1861:
1770:
452:
447:
412:
305:
3150:
3105:
2980:
2805:
2019:
1997:
1981:
1965:
1933:
1917:
1899:
1877:
1459:
in trace quantities, refines the grain structure of aluminum-based alloys.
944:
Usual base for brazing aluminum and its alloys. Embrittles ferrous alloys.
96:
2863:(2009). "Experimental survey on fluid brazing in ancient goldsmith' art".
106:
in that it does not involve melting the work pieces. Brazing differs from
3049:
2772:
2052:
1929:
1036:
Lowers melting point. Toxic. Produces toxic fumes, requires ventilation.
470:
134:
Brazing has many advantages over other metal-joining techniques, such as
3286:
3281:
3044:
2884:
2113:
1834:
1646:) that segregates at grain boundaries and cause intergranular failure.
1464:
1401:
1189:
722:
407:
215: in this section. Unsourced material may be challenged and removed.
27:
2902:
Fundamentals Of Modern
Manufacturing: Materials Processes, And Systems
442:
Crack in 90–10 Cu–Ni metal plate due to stresses during silver brazing
3276:
3271:
3204:
3181:
3095:
2123:
2068:
2038:
2009:
1812:
1791:
1730:
1669:
1560:
1496:
1443:
1349:
1242:
1230:
1164:
1095:
972:
764:
568:
535:
504:
403:
316:
107:
190:
3125:
2108:
1987:
1971:
1843:
Low hydrogen, AWS type 1, "exothermic generated atmospheres". 87% N
1799:
1601:
1535:
1341:
1141:
927:
810:
796:
690:
512:
399:
395:
115:
80:
20:
2664:
Supplies of
Cadmium Bearing Silver Solders Continue (2009-01-20).
2000:
and chromium alloys, cobalt alloys, tungsten alloys, and carbides.
967:
Excellent corrosion resistance. Very expensive. Wets most metals.
538:
is usually a brazing operation, with a filler rod made chiefly of
342:
118:) temperature while protected by a suitable atmosphere, usually a
3296:
3256:
3234:
3229:
3186:
3145:
3100:
2454:
1977:
1923:
1581:
1514:
1323:
1315:
1074:
996:
814:
800:
135:
123:
103:
2091:
in the United States recommends that exposure to these fumes is
38:
3224:
2949:
2742:
Joseph R. Davis, ASM International. Handbook
Committee (2001).
2520:
2031:
1886:
Dried, AWS type 3, "endothermic generated atmospheres. 73–75% N
1743:
1685:
1622:
1422:
1389:
1298:
1272:
1264:
1212:
1117:
1058:
874:
850:
787:
754:
676:
672:
640:
634:
629:
619:
572:
539:
523:
492:
363:
355:
3176:
2064:
2015:
1895:
1807:
1371:
697:
612:
500:
496:
92:
648:
using nickel, iron, copper, silicon, boron, phosphorus, etc.
1676:
When the brazing temperature is suitably high, brazing and
1617:
Promotes wetting of alumina ceramics by gold-based alloys.
1020:
949:
896:
737:
686:
625:
416:
122:. It then flows over the base metal (in a process known as
2694:"Lucas-Milhaupt SIL-FOS 18 Copper/Silver/Phosphorus Alloy"
1864:, AWS type 2, "endothermic generated atmospheres. 70–71% N
258:
A US Navy maintenance technician torch brazes a steel pipe
114:. The filler metal is brought slightly above its melting (
1828:
1061:. Improves wetting of many difficult-to-wet metals, e.g.
1041:
2640:"Welding Vs Soldering Vs Brazing - Learn The Difference"
666:
Unalloyed. Often noble metals – silver, gold, palladium.
3017:
2388:
2386:
431:
Brazing is widely used in the tool industry to fasten "
26:"Braze" redirects here. For the software company, see
2089:
National
Institute for Occupational Safety and Health
2383:
446:
Brazed hard metal joints are typically two to seven
2698:MatWeb – The Online Materials Information Resource
2418:"Guidelines for Selecting the Right Brazing Alloy"
1790:Unless brazing operations are contained within an
1680:can be done in a single operation simultaneously.
2276:"FAQ: What are the different methods of brazing?"
315:is a procedure where the heat is applied using a
3309:
1388:Lowers melting point. Can form hard and brittle
529:
2859:
1695:
2945:European Association for Brazing and Soldering
2554:
2022:chromium alloys, titanium, zirconium, hafnium.
172:Maximize the life of any fixtures or jigs used
3003:
2965:
2450:
2448:
2446:
2444:
2442:
2440:
2438:
1956:Cryogenic or purified (AWS type 6B). 70–99% N
1942:Cryogenic or purified (AWS type 6A). 70–99% N
1704:Brazing at the Gary Tubular Steel Plant, 1943
1692:structure, which possess further advantages.
567:Dip brazing is especially suited for brazing
2584:
2582:
2550:
2548:
2516:
2514:
2512:
2510:
2508:
2506:
2504:
2502:
2455:Christopher Corti; Richard Holliday (2009).
458:One special silver brazing method is called
169:Minimize filler metal/base metal interaction
2865:International Journal of Materials Research
2500:
2498:
2496:
2494:
2492:
2490:
2488:
2486:
2484:
2482:
3010:
2996:
2972:
2958:
2688:
2686:
2607:
2521:David M. Jacobson; Giles Humpston (2005).
2435:
2325:
2323:
2321:
2319:
2317:
2315:
1908:Dried, decarburizing, AWS type 4. 41–45% N
2804:
2579:
2545:
2367:"Flux Brazing Alloys | Lynch Metals, Inc"
2206:
2147:
2145:
2143:
2141:
2139:
231:Learn how and when to remove this message
166:Minimize any heat effects on the assembly
2918:
2834:
2710:
2590:"Ag slitiny bez Cd – speciální aplikace"
2479:
2404:
2392:
2329:
2306:
2302:
2300:
2298:
2296:
2262:
2234:
2230:
2228:
2226:
2194:
2182:
1699:
437:
366:with controlled atmosphere; and vacuum.
341:
253:
242:
79:
63:of all important aspects of the article.
2904:(2nd ed.). John Wiley & Sons.
2899:
2683:
2312:
2151:
740:-silver. Noble metals. Used in jewelry.
3310:
2136:
1267:for oxygen in the furnace atmosphere.
1207:volatilizing) and facilitate wetting.
59:Please consider expanding the lead to
2991:
2953:
2293:
2223:
2209:"Understanding Brazing Fundamentals"
2176:
499:filler rod coated with flux to join
213:adding citations to reliable sources
184:
32:
2527:. ASM International. pp. 71–.
1662:
579:
13:
2839:. London: Mills and Boon Limited.
2828:
2748:. ASM International. p. 311.
2666:"Strength of Silver Solder Joints"
2075:devices and components packaging.
337:
14:
3329:
2938:
1953:Nitrogen+hydrogen+carbon monoxide
1499:with some metals, e.g. nickel (Ni
546:
422:
2979:
2762:from the original on 2017-02-27.
2575:from the original on 2017-11-13.
2541:from the original on 2017-11-13.
2475:from the original on 2017-11-01.
652:Some brazes come in the form of
483:
300:
247:Brazing and soldering processes
189:
37:
2813:from the original on 2017-04-12
2785:
2766:
2735:
2716:
2704:
2672:from the original on 2011-08-12
2657:
2632:
2621:from the original on 2008-08-21
2410:
2398:
2359:
2335:
2083:Brazing may entail exposure to
1340:Lowers melting point. Can form
1314:Lowers melting point. Can form
290:Electron beam and laser brazing
200:needs additional citations for
51:may be too short to adequately
2458:Gold: Science and Applications
2420:. Silvaloy.com. Archived from
2268:
2256:
2240:
2200:
2188:
2173:. Deringer-Ney, April 29, 2014
2157:
562:
509:methylacetylene-propadiene gas
61:provide an accessible overview
1:
2129:
1818:
1046:structural, melting, wetting
1001:structural, wetting, melting
901:structural, melting, wetting
180:
2561:. CRC Press. pp. 272–.
2461:. CRC Press. pp. 184–.
2095:to levels below the allowed
1737:Care must be taken to avoid
1696:Interaction with base metals
595:
7:
2900:Groover, Mikell P. (2007).
2558:Industrial Brazing Practice
2164:"Joining Dissimilar Metals"
2102:
2045:
10:
3334:
2732:. GH Induction Atmospheres
583:
163:Minimize braze temperature
129:
25:
18:
3252:
3195:
3159:
3058:
3032:
2987:
2919:Schwartz, Mel M. (1987).
2078:
2055:to a substrate such as a
346:Furnace brazing schematic
309:automatic torch brazing.
3081:Electrohydraulic forming
2745:Copper and copper alloys
2617:. Azom.com. 2001-11-29.
2213:American Welding Society
1928:AWS type 5, also called
3086:Electromagnetic forming
2835:Fletcher, M.J. (1971).
2668:. www.cupalloys.co.uk.
2555:Philip Roberts (2003).
1785:
917:create voids. Prone to
803:. For brazing aluminum.
331:Automatic torch brazing
16:Metal-joining technique
3071:Casting (metalworking)
2806:10.26616/NIOSHPUB88110
2728:April 2, 2015, at the
1994:hydrogen embrittlement
1775:hydrogen embrittlement
1773:inclusions, and cause
1705:
1506:Fragmentation grenades
646:Amorphous brazing foil
586:List of brazing alloys
443:
347:
259:
251:
85:
3318:Brazing and soldering
3292:Tools and terminology
2923:. ASM International.
2778:July 8, 2011, at the
2524:Principles of Brazing
2280:The Welding Institute
2119:Petit chien à bélière
2057:printed circuit board
1912:, 17–19% CO, 38–40% H
1890:, 10–11% CO, 15–16% H
1703:
836:corrosion resistance
441:
345:
324:Machine torch brazing
257:
246:
102:Brazing differs from
83:
3121:Progressive stamping
2087:chemical fumes. The
1936:and chromium alloys.
1872:, 9–10% CO, 14–15% H
1354:structural, melting
1122:structural, wetting
1025:structural, melting
954:structural, wetting
855:structural, wetting
704:cracking by ammonia.
313:Manual torch brazing
249:classification chart
209:improve this article
3197:Finishing processes
2877:2009IJMR..100...81C
1796:reducing atmosphere
1606:structural, active
1565:structural, active
1540:structural, active
932:structural, active
823:
530:Cast iron "welding"
478:cathodic protection
2885:10.3139/146.101783
2713:, pp. 271–279
2407:, pp. 163–185
2395:, pp. 131–160
2332:, pp. 199–222
2309:, pp. 189–198
2197:, pp. 118–119
2169:2014-03-04 at the
2154:, pp. 746–748
2025:Noble gas+hydrogen
1905:Combusted fuel gas
1898:, medium and high
1883:Combusted fuel gas
1858:Combusted fuel gas
1840:Combusted fuel gas
1798:environment (i.e.
1779:Oxygen-free copper
1739:galvanic corrosion
1706:
919:selective leaching
821:
444:
348:
281:Resistance brazing
260:
252:
86:
3305:
3304:
3248:
3247:
3160:Joining processes
3091:Explosive forming
3059:Forming processes
2930:978-0-87170-246-3
2911:978-81-265-1266-9
2723:The Brazing Guide
2615:"Ceramic Brazing"
2568:978-0-203-48857-7
2534:978-1-61503-104-7
2468:978-1-4200-6526-8
2371:Lynch Metals, Inc
1939:Nitrogen+hydrogen
1851:, 5-1% CO, 5-1% H
1650:
1649:
1376:melting, wetting
1328:melting, wetting
1277:melting, wetting
822:Role of elements
534:The "welding" of
274:Induction brazing
241:
240:
233:
78:
77:
3325:
3027:
3012:
3005:
2998:
2989:
2988:
2974:
2967:
2960:
2951:
2950:
2934:
2915:
2896:
2850:
2822:
2821:
2819:
2818:
2808:
2789:
2783:
2770:
2764:
2763:
2739:
2733:
2720:
2714:
2708:
2702:
2701:
2690:
2681:
2680:
2678:
2677:
2661:
2655:
2654:
2652:
2651:
2636:
2630:
2629:
2627:
2626:
2611:
2605:
2604:
2602:
2601:
2592:. Archived from
2586:
2577:
2576:
2552:
2543:
2542:
2518:
2477:
2476:
2452:
2433:
2432:
2430:
2429:
2414:
2408:
2402:
2396:
2390:
2381:
2380:
2378:
2377:
2363:
2357:
2356:
2354:
2353:
2339:
2333:
2327:
2310:
2304:
2291:
2290:
2288:
2286:
2272:
2266:
2265:, pp. 24–37
2260:
2254:
2244:
2238:
2237:, pp. 20–24
2232:
2221:
2220:
2215:. Archived from
2204:
2198:
2192:
2186:
2180:
2174:
2161:
2155:
2149:
2003:Inorganic vapors
1756:nickel silicides
1663:Melting behavior
1291:titanium nitride
1067:tungsten carbide
1063:stainless steels
842:incompatibility
824:
820:
602:Aluminum-silicon
580:Filler materials
491:is the use of a
474:
473:
464:
463:
284:Infrared brazing
236:
229:
225:
222:
216:
193:
185:
152:capillary action
112:capillary action
84:Brazing practice
73:
70:
64:
41:
33:
3333:
3332:
3328:
3327:
3326:
3324:
3323:
3322:
3308:
3307:
3306:
3301:
3244:
3191:
3155:
3116:Press hardening
3054:
3028:
3026:, and finishing
3018:
3016:
2983:
2978:
2941:
2931:
2912:
2861:Andrea Cagnetti
2847:
2831:
2829:Further reading
2826:
2825:
2816:
2814:
2791:
2790:
2786:
2780:Wayback Machine
2773:Solder Preforms
2771:
2767:
2756:
2740:
2736:
2730:Wayback Machine
2721:
2717:
2709:
2705:
2692:
2691:
2684:
2675:
2673:
2662:
2658:
2649:
2647:
2638:
2637:
2633:
2624:
2622:
2613:
2612:
2608:
2599:
2597:
2588:
2587:
2580:
2569:
2553:
2546:
2535:
2519:
2480:
2469:
2453:
2436:
2427:
2425:
2416:
2415:
2411:
2403:
2399:
2391:
2384:
2375:
2373:
2365:
2364:
2360:
2351:
2349:
2341:
2340:
2336:
2328:
2313:
2305:
2294:
2284:
2282:
2274:
2273:
2269:
2261:
2257:
2245:
2241:
2233:
2224:
2207:Alan Belohlav.
2205:
2201:
2193:
2189:
2181:
2177:
2171:Wayback Machine
2162:
2158:
2150:
2137:
2132:
2105:
2081:
2048:
1963:
1959:
1949:
1945:
1915:
1911:
1893:
1889:
1875:
1871:
1867:
1854:
1850:
1846:
1821:
1788:
1698:
1665:
1645:
1641:
1553:silicon nitride
1502:
1490:
1482:
1448:trace additive
1427:trace additive
1406:trace additive
1250:
1235:gamma radiation
1100:trace additive
1079:trace additive
985:very expensive
962:very expensive
588:
582:
565:
549:
532:
486:
469:
468:
461:
460:
425:
392:Vacuum furnaces
378:Continuous type
352:unskilled labor
340:
338:Furnace brazing
303:
287:Blanket brazing
270:Furnace brazing
237:
226:
220:
217:
206:
194:
183:
132:
74:
68:
65:
58:
46:This article's
42:
31:
24:
17:
12:
11:
5:
3331:
3321:
3320:
3303:
3302:
3300:
3299:
3294:
3289:
3284:
3279:
3274:
3269:
3264:
3259:
3253:
3250:
3249:
3246:
3245:
3243:
3242:
3237:
3232:
3227:
3222:
3220:Mass finishing
3217:
3212:
3207:
3201:
3199:
3193:
3192:
3190:
3189:
3184:
3179:
3174:
3169:
3163:
3161:
3157:
3156:
3154:
3153:
3148:
3143:
3138:
3133:
3128:
3123:
3118:
3113:
3108:
3103:
3098:
3093:
3088:
3083:
3078:
3073:
3068:
3062:
3060:
3056:
3055:
3053:
3052:
3047:
3042:
3036:
3034:
3030:
3029:
3015:
3014:
3007:
3000:
2992:
2985:
2984:
2977:
2976:
2969:
2962:
2954:
2948:
2947:
2940:
2939:External links
2937:
2936:
2935:
2929:
2916:
2910:
2897:
2857:
2854:
2851:
2845:
2837:Vacuum Brazing
2830:
2827:
2824:
2823:
2784:
2765:
2754:
2734:
2715:
2703:
2682:
2656:
2631:
2606:
2578:
2567:
2544:
2533:
2478:
2467:
2434:
2409:
2397:
2382:
2358:
2347:www.lytron.com
2334:
2311:
2292:
2267:
2255:
2239:
2222:
2219:on 2014-02-27.
2199:
2187:
2175:
2156:
2134:
2133:
2131:
2128:
2127:
2126:
2121:
2116:
2111:
2104:
2101:
2097:exposure limit
2080:
2077:
2073:optoelectronic
2047:
2044:
2043:
2042:
2034:
2029:
2026:
2023:
2012:
2007:
2004:
2001:
1990:
1985:
1974:
1969:
1961:
1957:
1954:
1951:
1947:
1943:
1940:
1937:
1926:
1921:
1913:
1909:
1906:
1903:
1891:
1887:
1884:
1881:
1873:
1869:
1865:
1859:
1856:
1852:
1848:
1844:
1841:
1838:
1831:
1820:
1817:
1787:
1784:
1697:
1694:
1678:heat treatment
1664:
1661:
1648:
1647:
1643:
1639:
1636:
1634:
1632:
1630:
1628:
1625:
1619:
1618:
1615:
1613:
1611:
1609:
1607:
1604:
1598:
1597:
1595:
1593:
1591:
1589:
1587:
1584:
1578:
1577:
1574:
1572:
1570:
1568:
1566:
1563:
1557:
1556:
1549:
1547:
1545:
1543:
1541:
1538:
1532:
1531:
1528:
1526:
1524:
1522:
1520:
1517:
1511:
1510:
1500:
1493:
1488:
1480:
1476:
1474:
1472:
1470:
1467:
1461:
1460:
1457:
1455:
1453:
1451:
1449:
1446:
1440:
1439:
1436:
1434:
1432:
1430:
1428:
1425:
1419:
1418:
1415:
1413:
1411:
1409:
1407:
1404:
1398:
1397:
1394:neutron poison
1386:
1383:
1381:
1379:
1377:
1374:
1368:
1367:
1364:
1362:
1359:
1357:
1355:
1352:
1346:
1345:
1338:
1335:
1333:
1331:
1329:
1326:
1320:
1319:
1312:
1310:
1308:
1306:
1304:
1301:
1295:
1294:
1287:
1285:
1282:
1280:
1278:
1275:
1269:
1268:
1261:
1259:
1257:
1255:
1252:
1248:
1245:
1239:
1238:
1227:
1225:
1223:
1220:
1218:
1215:
1209:
1208:
1204:
1202:
1200:
1197:
1195:
1192:
1186:
1185:
1181:
1179:
1176:
1173:
1170:
1167:
1161:
1160:
1156:
1154:
1152:
1149:
1147:
1144:
1138:
1137:
1133:
1130:
1128:
1125:
1123:
1120:
1114:
1113:
1110:
1107:
1105:
1103:
1101:
1098:
1092:
1091:
1088:
1086:
1084:
1082:
1080:
1077:
1071:
1070:
1055:
1053:
1051:
1049:
1047:
1044:
1038:
1037:
1034:
1032:
1030:
1028:
1026:
1023:
1017:
1016:
1012:
1009:
1007:
1005:
1002:
999:
993:
992:
988:
986:
983:
980:
978:
975:
969:
968:
965:
963:
960:
957:
955:
952:
946:
945:
942:
939:
937:
935:
933:
930:
924:
923:
914:
911:
908:
905:
902:
899:
893:
892:
889:
886:
884:
882:
880:
877:
871:
870:
866:
864:
861:
859:
856:
853:
847:
846:
843:
840:
837:
834:
831:
828:
819:
818:
807:
804:
794:
791:
785:
782:
778:
775:
771:
768:
761:
758:
751:
748:
744:
741:
735:
732:
729:
726:
719:
716:
708:
705:
701:
694:
683:
680:
670:
667:
664:
650:
649:
643:
638:
632:
623:
616:
609:
606:
603:
581:
578:
564:
561:
548:
547:Vacuum brazing
545:
531:
528:
485:
482:
424:
423:Silver brazing
421:
339:
336:
302:
299:
295:
294:
291:
288:
285:
282:
279:
276:
271:
268:
239:
238:
197:
195:
188:
182:
179:
174:
173:
170:
167:
164:
131:
128:
76:
75:
55:the key points
45:
43:
36:
15:
9:
6:
4:
3:
2:
3330:
3319:
3316:
3315:
3313:
3298:
3295:
3293:
3290:
3288:
3285:
3283:
3280:
3278:
3275:
3273:
3270:
3268:
3265:
3263:
3260:
3258:
3255:
3254:
3251:
3241:
3238:
3236:
3233:
3231:
3228:
3226:
3223:
3221:
3218:
3216:
3215:Heat treating
3213:
3211:
3208:
3206:
3203:
3202:
3200:
3198:
3194:
3188:
3185:
3183:
3180:
3178:
3175:
3173:
3170:
3168:
3165:
3164:
3162:
3158:
3152:
3149:
3147:
3144:
3142:
3139:
3137:
3134:
3132:
3129:
3127:
3124:
3122:
3119:
3117:
3114:
3112:
3109:
3107:
3104:
3102:
3099:
3097:
3094:
3092:
3089:
3087:
3084:
3082:
3079:
3077:
3074:
3072:
3069:
3067:
3064:
3063:
3061:
3057:
3051:
3048:
3046:
3043:
3041:
3038:
3037:
3035:
3031:
3025:
3021:
3013:
3008:
3006:
3001:
2999:
2994:
2993:
2990:
2986:
2982:
2975:
2970:
2968:
2963:
2961:
2956:
2955:
2952:
2946:
2943:
2942:
2932:
2926:
2922:
2917:
2913:
2907:
2903:
2898:
2894:
2890:
2886:
2882:
2878:
2874:
2870:
2866:
2862:
2858:
2855:
2852:
2848:
2846:0-263-51708-X
2842:
2838:
2833:
2832:
2812:
2807:
2802:
2798:
2794:
2788:
2782:. AMETEK.Inc.
2781:
2777:
2774:
2769:
2761:
2757:
2755:0-87170-726-8
2751:
2747:
2746:
2738:
2731:
2727:
2724:
2719:
2712:
2711:Schwartz 1987
2707:
2699:
2695:
2689:
2687:
2671:
2667:
2660:
2645:
2644:Welder Choice
2641:
2635:
2620:
2616:
2610:
2596:on 2016-04-20
2595:
2591:
2585:
2583:
2574:
2570:
2564:
2560:
2559:
2551:
2549:
2540:
2536:
2530:
2526:
2525:
2517:
2515:
2513:
2511:
2509:
2507:
2505:
2503:
2501:
2499:
2497:
2495:
2493:
2491:
2489:
2487:
2485:
2483:
2474:
2470:
2464:
2460:
2459:
2451:
2449:
2447:
2445:
2443:
2441:
2439:
2424:on 2010-10-07
2423:
2419:
2413:
2406:
2405:Schwartz 1987
2401:
2394:
2393:Schwartz 1987
2389:
2387:
2372:
2368:
2362:
2348:
2344:
2338:
2331:
2330:Schwartz 1987
2326:
2324:
2322:
2320:
2318:
2316:
2308:
2307:Schwartz 1987
2303:
2301:
2299:
2297:
2281:
2277:
2271:
2264:
2263:Schwartz 1987
2259:
2253:
2252:0-87171-624-0
2249:
2243:
2236:
2235:Schwartz 1987
2231:
2229:
2227:
2218:
2214:
2210:
2203:
2196:
2195:Schwartz 1987
2191:
2184:
2183:Schwartz 1987
2179:
2172:
2168:
2165:
2160:
2153:
2148:
2146:
2144:
2142:
2140:
2135:
2125:
2122:
2120:
2117:
2115:
2112:
2110:
2107:
2106:
2100:
2098:
2094:
2090:
2086:
2076:
2074:
2070:
2066:
2062:
2058:
2054:
2041:applications.
2040:
2035:
2033:
2030:
2027:
2024:
2021:
2020:carbon steels
2017:
2013:
2011:
2008:
2005:
2002:
1999:
1998:carbon steels
1995:
1991:
1989:
1986:
1983:
1982:carbon steels
1979:
1975:
1973:
1970:
1967:
1966:carbon steels
1955:
1952:
1941:
1938:
1935:
1934:carbon steels
1931:
1927:
1925:
1922:
1919:
1918:carbon steels
1907:
1904:
1901:
1900:carbon steels
1897:
1885:
1882:
1879:
1878:carbon steels
1863:
1862:Decarburizing
1860:
1857:
1842:
1839:
1836:
1832:
1830:
1827:
1826:
1825:
1816:
1814:
1809:
1805:
1801:
1797:
1793:
1783:
1780:
1776:
1772:
1771:cuprous oxide
1767:
1763:
1759:
1757:
1751:
1747:
1745:
1740:
1735:
1732:
1727:
1723:
1719:
1716:
1713:
1710:
1702:
1693:
1689:
1687:
1681:
1679:
1674:
1672:
1671:
1660:
1657:
1654:
1637:
1635:
1633:
1631:
1629:
1626:
1624:
1621:
1620:
1616:
1614:
1612:
1610:
1608:
1605:
1603:
1600:
1599:
1596:
1594:
1592:
1590:
1588:
1585:
1583:
1580:
1579:
1575:
1573:
1571:
1569:
1567:
1564:
1562:
1559:
1558:
1554:
1550:
1548:
1546:
1544:
1542:
1539:
1537:
1534:
1533:
1529:
1527:
1525:
1523:
1521:
1518:
1516:
1513:
1512:
1507:
1498:
1494:
1492:, Ni, Fe, Co
1491:
1484:
1477:
1475:
1473:
1471:
1468:
1466:
1463:
1462:
1458:
1456:
1454:
1452:
1450:
1447:
1445:
1442:
1441:
1437:
1435:
1433:
1431:
1429:
1426:
1424:
1421:
1420:
1416:
1414:
1412:
1410:
1408:
1405:
1403:
1400:
1399:
1395:
1391:
1387:
1384:
1382:
1380:
1378:
1375:
1373:
1370:
1369:
1365:
1363:
1360:
1358:
1356:
1353:
1351:
1348:
1347:
1343:
1339:
1336:
1334:
1332:
1330:
1327:
1325:
1322:
1321:
1317:
1313:
1311:
1309:
1307:
1305:
1302:
1300:
1297:
1296:
1292:
1288:
1286:
1283:
1281:
1279:
1276:
1274:
1271:
1270:
1266:
1262:
1260:
1258:
1256:
1253:
1246:
1244:
1241:
1240:
1236:
1232:
1228:
1226:
1224:
1221:
1219:
1216:
1214:
1211:
1210:
1205:
1203:
1201:
1198:
1196:
1193:
1191:
1188:
1187:
1182:
1180:
1177:
1174:
1171:
1168:
1166:
1163:
1162:
1157:
1155:
1153:
1150:
1148:
1145:
1143:
1140:
1139:
1134:
1131:
1129:
1126:
1124:
1121:
1119:
1116:
1115:
1111:
1108:
1106:
1104:
1102:
1099:
1097:
1094:
1093:
1089:
1087:
1085:
1083:
1081:
1078:
1076:
1073:
1072:
1068:
1064:
1060:
1056:
1054:
1052:
1050:
1048:
1045:
1043:
1040:
1039:
1035:
1033:
1031:
1029:
1027:
1024:
1022:
1019:
1018:
1013:
1010:
1008:
1006:
1003:
1000:
998:
995:
994:
989:
987:
984:
981:
979:
976:
974:
971:
970:
966:
964:
961:
958:
956:
953:
951:
948:
947:
943:
940:
938:
936:
934:
931:
929:
926:
925:
920:
915:
912:
909:
906:
903:
900:
898:
895:
894:
890:
887:
885:
883:
881:
878:
876:
873:
872:
869:gold-copper.
867:
865:
862:
860:
857:
854:
852:
849:
848:
844:
841:
838:
835:
832:
829:
826:
825:
816:
812:
808:
806:Active alloys
805:
802:
798:
795:
792:
789:
786:
783:
779:
776:
772:
769:
766:
762:
759:
756:
752:
749:
745:
742:
739:
736:
733:
730:
727:
724:
720:
717:
714:
709:
706:
702:
699:
695:
692:
688:
684:
681:
678:
674:
671:
668:
665:
662:
661:
660:
657:
655:
647:
644:
642:
639:
636:
633:
631:
627:
624:
621:
617:
614:
611:Copper-zinc (
610:
608:Copper-silver
607:
604:
601:
600:
599:
597:
592:
587:
577:
574:
570:
560:
556:
553:
544:
541:
537:
527:
525:
520:
516:
514:
510:
506:
502:
498:
494:
490:
489:Braze welding
484:Braze welding
481:
479:
475:
472:
465:
456:
454:
453:copper-nickel
449:
440:
436:
434:
429:
420:
418:
414:
409:
405:
401:
397:
393:
389:
386:
382:
379:
375:
372:
367:
365:
360:
357:
353:
344:
335:
332:
328:
325:
321:
318:
314:
310:
307:
301:Torch brazing
298:
293:Braze welding
292:
289:
286:
283:
280:
277:
275:
272:
269:
267:Torch brazing
266:
265:
264:
256:
250:
245:
235:
232:
224:
214:
210:
204:
203:
198:This section
196:
192:
187:
186:
178:
171:
168:
165:
162:
161:
160:
156:
153:
147:
143:
141:
137:
127:
125:
121:
117:
113:
109:
105:
100:
98:
94:
90:
82:
72:
62:
56:
54:
49:
44:
40:
35:
34:
29:
22:
3166:
3151:Tube bending
3106:Hydroforming
2981:Metalworking
2920:
2901:
2871:(1): 81–85.
2868:
2864:
2836:
2815:. Retrieved
2796:
2787:
2768:
2744:
2737:
2718:
2706:
2697:
2674:. Retrieved
2659:
2648:. Retrieved
2646:. 2021-03-01
2643:
2634:
2623:. Retrieved
2609:
2598:. Retrieved
2594:the original
2557:
2523:
2457:
2426:. Retrieved
2422:the original
2412:
2400:
2374:. Retrieved
2370:
2361:
2350:. Retrieved
2346:
2337:
2283:. Retrieved
2279:
2270:
2258:
2242:
2217:the original
2212:
2202:
2190:
2178:
2159:
2152:Groover 2007
2082:
2053:silicon dies
2049:
2028:AWS type 9A.
1822:
1789:
1768:
1764:
1760:
1752:
1748:
1736:
1728:
1724:
1720:
1717:
1714:
1711:
1707:
1690:
1682:
1675:
1668:
1666:
1658:
1655:
1651:
845:description
712:
658:
653:
651:
618:Copper-tin (
593:
589:
566:
557:
554:
550:
533:
521:
517:
488:
487:
467:
459:
457:
445:
430:
426:
391:
390:
384:
383:
377:
376:
370:
368:
361:
349:
330:
329:
323:
322:
312:
311:
304:
296:
261:
227:
218:
207:Please help
202:verification
199:
175:
157:
148:
144:
133:
101:
97:filler metal
88:
87:
69:January 2020
66:
50:
48:lead section
3262:Fabrication
3210:Galvanizing
3050:Sheet metal
3040:Fabrication
3024:fabrication
2797:www.cdc.gov
2285:27 December
2185:, p. 3
1930:forming gas
1847:, 11–12% CO
1519:deoxidizer
1469:deoxidizer
1233:, a potent
1217:structural
1194:structural
1169:structural
1146:structural
977:structural
879:structural
833:volatility
713:Ag-Cu-Zn-Cd
691:hallmarking
663:Pure metals
563:Dip brazing
471:pin brazing
385:Retort-type
278:Dip brazing
221:August 2010
3282:Metallurgy
3225:Patination
3045:Piece work
2817:2017-04-11
2676:2010-07-26
2650:2021-03-03
2625:2010-07-26
2600:2016-04-07
2428:2010-07-26
2376:2017-12-27
2352:2017-12-27
2130:References
2114:CuproBraze
2093:controlled
2069:rectifiers
2061:lead wires
1819:Atmosphere
1813:phosphides
1731:phosphides
1497:phosphides
1465:Phosphorus
1402:Mischmetal
1361:expensive
1284:expensive
1247:volatile O
1190:Molybdenum
982:excellent
959:excellent
863:expensive
723:phosphorus
584:See also:
462:pinbrazing
433:hard metal
415:(10 to 10
408:refractory
181:Techniques
28:Braze, Inc
3277:Machining
3272:Jewellery
3240:Polishing
3205:Anodizing
3182:Soldering
3096:Extrusion
2893:137786674
2124:Soldering
2085:hazardous
2039:aerospace
2010:Noble gas
1960:, 2–20% H
1946:, 1–30% H
1868:, 5–6% CO
1670:liquation
1627:impurity
1561:Zirconium
1444:Strontium
1350:Germanium
1342:silicides
1254:volatile
1243:Magnesium
1237:emitter.
1231:cobalt-60
1172:volatile
1165:Manganese
1096:Beryllium
1004:volatile
973:Palladium
904:volatile
858:volatile
774:pressure.
765:Palladium
569:aluminium
536:cast iron
522:Carbide,
505:acetylene
404:zirconium
317:gas flame
108:soldering
53:summarize
3312:Category
3287:Smithing
3177:Riveting
3172:Crimping
3141:Spinning
3126:Punching
3111:Stamping
2811:Archived
2799:. 1988.
2776:Archived
2760:Archived
2726:Archived
2670:Archived
2619:Archived
2573:Archived
2539:Archived
2473:Archived
2167:Archived
2109:Braze-on
2103:See also
2046:Preforms
2014:Usually
1988:Hydrogen
1978:nitrides
1972:Nitrogen
1806:such as
1800:Nitrogen
1602:Vanadium
1536:Titanium
1316:carbides
1303:melting
1142:Chromium
928:Aluminum
888:ammonia
827:element
815:ceramics
811:graphite
797:Aluminum
707:Ag-Cu-Zn
654:trifoils
596:preforms
513:MAPP gas
400:titanium
396:aluminum
116:liquidus
21:Braising
3297:Welding
3267:Forming
3257:Casting
3235:Plating
3230:Peening
3187:Welding
3167:Brazing
3146:Swaging
3136:Sinking
3131:Rolling
3101:Forging
3076:Drawing
3066:Coining
3033:General
3020:Forming
2921:Brazing
2873:Bibcode
1924:Ammonia
1744:solders
1686:solders
1586:active
1582:Hafnium
1515:Lithium
1390:borides
1324:Silicon
1251:getter
1075:Bismuth
997:Cadmium
801:silicon
728:Ag-Cu-P
721:Copper-
696:Cu-Zn (
685:Silver-
413:pascals
136:welding
130:Process
124:wetting
104:welding
89:Brazing
2927:
2908:
2891:
2843:
2752:
2565:
2531:
2465:
2250:
2079:Safety
2065:diodes
2032:Vacuum
1623:Sulfur
1423:Cerium
1299:Carbon
1273:Indium
1265:getter
1213:Cobalt
1178:cheap
1132:Zn, S
1118:Nickel
1109:toxic
1059:bronze
1011:toxic
910:cheap
875:Copper
851:Silver
788:Cobalt
755:Nickel
677:copper
673:Silver
641:Silver
635:Nickel
630:silver
620:bronze
605:Copper
573:slurry
540:nickel
524:cermet
493:bronze
364:retort
356:vacuum
2889:S2CID
2016:argon
1896:Monel
1835:scale
1808:borax
1802:), a
1792:inert
1372:Boron
1222:good
1199:good
1175:good
1151:high
1127:high
839:cost
830:role
793:Al-Si
763:Gold-
760:Au-Pd
753:Gold-
750:Au-Ni
743:Au-Cu
734:Au-Ag
698:brass
682:Ag-Zn
669:Ag-Cu
637:alloy
613:brass
501:steel
497:brass
371:batch
306:Torch
93:metal
91:is a
2925:ISBN
2906:ISBN
2841:ISBN
2750:ISBN
2563:ISBN
2529:ISBN
2463:ISBN
2287:2017
2248:ISBN
1804:flux
1786:Flux
1065:and
1021:Lead
950:Gold
907:low
897:Zinc
738:Gold
718:Cu-P
687:zinc
626:Gold
448:mils
417:Torr
402:and
140:clad
120:flux
2881:doi
2869:100
2801:doi
1829:Air
1794:or
1385:Ni
1337:Ni
1042:Tin
941:Fe
913:Ni
813:or
507:or
495:or
466:or
211:by
3314::
3022:,
2887:.
2879:.
2867:.
2809:.
2795:.
2758:.
2696:.
2685:^
2642:.
2581:^
2571:.
2547:^
2537:.
2481:^
2471:.
2437:^
2385:^
2369:.
2345:.
2314:^
2295:^
2278:.
2225:^
2211:.
2138:^
2099:.
2071:,
2067:,
1746:.
1688:.
1487:SO
1485:,
1293:.
784:Co
777:Ni
770:Pd
455:.
398:,
369:A
3011:e
3004:t
2997:v
2973:e
2966:t
2959:v
2933:.
2914:.
2895:.
2883::
2875::
2849:.
2820:.
2803::
2700:.
2679:.
2653:.
2628:.
2603:.
2431:.
2379:.
2355:.
2289:.
1984:.
1968:.
1962:2
1958:2
1948:2
1944:2
1920:.
1914:2
1910:2
1902:.
1892:2
1888:2
1880:.
1874:2
1870:2
1866:2
1853:2
1849:2
1845:2
1644:2
1642:S
1640:3
1501:3
1489:2
1483:S
1481:2
1479:H
1249:2
817:.
799:-
700:)
675:-
628:-
622:)
615:)
511:(
234:)
228:(
223:)
219:(
205:.
71:)
67:(
57:.
30:.
23:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.