707:
684:
158:
149:
1152:
1028:
923:
43:
2392:
1231:. Severe bleeds occurred in 4.4 and 4.7% of patients respectively depending on the infusion rate (0.5 μg/kg/min vs. 0.75 μg/kg/min). A few cases of death due to severe bleeding events attributable to drug therapy were reported. No cases of hemorrhagic stroke were seen. Thrombocytopenia of unknown origin (allergic reaction?) was also noticed in 0.2% of patients.
824:
InChI=1S/C35H49N11O9S2/c36-30(51)25-18-57-56-13-10-27(47)42-22(8-3-4-11-39-35(37)38)31(52)41-17-28(48)43-23(15-29(49)50)32(53)44-24(14-19-16-40-21-7-2-1-6-20(19)21)34(55)46-12-5-9-26(46)33(54)45-25/h1-2,6-7,16,22-26,40H,3-5,8-15,17-18H2,(H2,36,51)(H,41,52)(H,42,47)(H,43,48)(H,44,53)(H,45,54)(H,49,50)(H4,37,38,39)/t22-,23-,24-,25-,26-/m0/s1
1011:. Additionally, the usual supportive treatment consisting of applications of nitrates, beta-blockers, opioid analgesics and/or benzodiazepines should be employed as indicated. Angiographic evaluation and other intensive diagnostic procedures may be considered a first line task before initiating therapy with eptifibatide.
782:
1256:
Sometimes the treating physicians require the patient after discharge from hospital to continue treatment with aspirin or clopidogrel for a few weeks, some months or even for life (as usually is the case with aspirin) to prevent recurrence of symptoms, development of myocardial infarction and/or
1247:
Eptifibatide was licensed due to the positive results of the so-called PURSUIT study encompassing 10,948 patients. In this study all patients had experienced either unstable angina or a non-ST-segment-elevation myocardial infarction. Significantly fewer patients developed a myocardial infarction
1238:
was seen frequently (6%). Cardiovascular failure was also frequent (2%) as were serious arrhythmias (ventricular fibrillation 1.5%, atrial fibrillation 6%). Severe allergic (anaphylactic) reactions occurred in almost 0.2% of patients. These reactions can be life-threatening and may be due to the
1257:
death related to cardiovascular disease. This advice should be strictly followed. Eptifibatide is one of very many antiplatelet drugs that all have different consequences on the platelet's activity. Eptifibatide has been shown to have salutary effects for patients with Covid related thrombosis.
991:
or non-ST-segment-elevation (e.g., non-Q-wave) myocardial infarction (i.e., non-ST-segment elevation acute coronary syndromes) both in patients who are to receive non surgery (conservative) medical treatment and those undergoing
1134:
Geriatric patients : No differences in side effects compared with younger patients have been seen. Nevertheless, geriatric patients should be very closely observed for bleeding and other side-effects.
1223:. Bleeding occurred as well at sites of clinical intervention (local sites) as at other sites (systemically) like urogenital bleeds. Sometimes, these events were severe enough to require
265:
335:
1014:
The drug should exclusively be used in hospitalized patients both because of the serious degree of patients' illness and because of the possible side-effects of eptifibatide.
1212:
People receiving eptifibatide are typically seriously ill and most of them are concomitantly treated with other drugs known to have the potential to cause significant
1098: : Eptifibatide undergoes kidney elimination. In such patients with chronic kidney disease where a glycoprotein IIb/IIIa inhibitor is likely to provide benefit,
2437:
1092: : The drug is contraindicated in patients with platelet counts of less than 100,000 per μL because no clinical experience exists regarding such patients.
290:
1109:
Current bleeding tendencies or abnormally prolonged coagulation parameters observed within 30 days before starting therapy with eptifibatide is intended.
231:
1248:
under therapy with eptifibatide. Death rates showed a tendency in favor of eptifibatide, but this superiority was not statistically significant.
1420:
180:
796:
2427:
2133:
1536:
1531:
2432:
2412:
2232:
1470:
1239:
peptide character of eptifibatide. Other side effects were rare and mild in nature and may not be connected to eptifibatide therapy.
1562:
1131:
Lactation : No human data exists. Breast-feeding should be avoided during treatment in order to prevent damage to the newborn.
1448:
2346:
1313:
1386:
107:
79:
1137:
Pediatric patients : Eptifibatide is not indicated in patients below 18 years of age, because no experience exists.
841:
248:
993:
1717:
1199:
1075:
970:
816:
261:
126:
1181:
1057:
952:
86:
2363:
1513:
1282:
2327:
1735:
1708:
867:
441:
320:
212:
2119:
1662:
1177:
1053:
948:
64:
2093:
1112:
Coagulation parameters such as ACT, aPTT, TT, and PT should be followed closely during therapy and afterwards.
532:
93:
1463:
1173:
1049:
944:
60:
901:. The drug is the third inhibitor of GPIIb/IIIa that has found broad acceptance after the specific antibody
1946:
889:
702:
583:
2037:
1757:
1216:. Therefore, not all side effects listed as follows may be attributable to eptifibatide treatment alone:
663:
75:
2382:
1270:
894:
859:
652:
17:
2085:
1269:
by a team led by Robert M. Scarborough and David
Phillips, at COR Therapeutics which was acquired by
226:
1408:
1128:
Pregnancy : No experience exists. Pregnant patients should be treated only when clearly needed.
2417:
1456:
1162:
1038:
933:
679:
1428:
2351:
1166:
1042:
937:
276:
170:
53:
1478:
1700:
1624:
1095:
632:
398:
572:
2228:
1303:
1213:
984:
523:
1437:
804:
14CCCN1C(=O)(Cc2cc3ccccc23)NC(=O)(CC(=O)O)NC(=O)CNC(=O)(CCCCNC(=N)N)NC(=O)CCSSC(C(N)=O)NC4=O
487:
2422:
2290:
1869:
1824:
1338:"Successful Use of Glycoprotein IIb/IIIa Inhibitor Involving Severely Ill COVID-19 Patient"
592:
408:
100:
8:
2442:
1680:
706:
683:
478:
283:
1971:
1362:
1337:
983:
Eptifibatide is used to reduce the risk of acute cardiac ischemic events (death and/or
2215:
1986:
1976:
1966:
1961:
1956:
1931:
1504:
1491:
1483:
1367:
1309:
1224:
863:
361:
348:
204:
190:
2189:
2020:
1981:
1951:
1357:
1349:
1089:
719:
512:
380:
303:
2396:
1993:
988:
871:
388:
2357:
1884:
1570:
1479:
1266:
157:
2406:
2314:
2284:
1874:
1859:
1854:
1815:
1487:
695:
140:
2304:
2274:
2252:
2247:
2220:
2169:
2148:
1998:
1937:
1893:
1770:
1713:
1652:
1647:
1628:
1495:
1371:
1122:
874:
298:
1305:
Heterocyclic
Scaffolds II: Indoles: Synthesis, Properties and Applications
2174:
2102:
2071:
2003:
1904:
1898:
1888:
1791:
1585:
1575:
1353:
1235:
1004:
883:
878:
198:
148:
31:
552:
2279:
2196:
2179:
2164:
2143:
2128:
2123:
2066:
2051:
2025:
2015:
2010:
1864:
1833:
1765:
1745:
1723:
1612:
1596:
1592:
1541:
758:
563:
897:-mimetics and reversibly binds to platelets. Eptifibatide has a short
2267:
2262:
2242:
2237:
2184:
2138:
2112:
2107:
2056:
1914:
1837:
1801:
1775:
1740:
1728:
1690:
1685:
1675:
1637:
1607:
1602:
1580:
1546:
1521:
1099:
906:
902:
898:
498:
421:
184:
1151:
1027:
922:
42:
2061:
2046:
1879:
1850:
1841:
1829:
1796:
1642:
1220:
612:
543:
243:
781:
2332:
2322:
2299:
2097:
1926:
1670:
1387:"Robert Scarborough Jr. -- helped discover important heart drugs"
1115:
1008:
1000:
230:
1227:
of blood or plasma concentrates to stop bleeding and counteract
2294:
1228:
1103:
643:
426:
1556:
772:
623:
887:) found in the venom of the southeastern pygmy rattlesnake (
668:
2201:
603:
1219:
The major adverse event in the PURSUIT study was severe
1433:(information on the biological origin of eptifibatide)
2380:
1017:
862:, also co-promoted by Schering-Plough/Essex), is an
531:
67:. Unsourced material may be challenged and removed.
2438:Drugs developed by Takeda Pharmaceutical Company
2404:
1335:
511:
449:N-(Aminoiminomethyl)-N-(3-mercapto-1-oxopropyl)-
486:
260:
1464:
1007:and (low molecular weight or unfractionated)
247:
1180:. Unsourced material may be challenged and
1056:. Unsourced material may be challenged and
951:. Unsourced material may be challenged and
1471:
1457:
1336:Merrill PJ, Bradburne RM (December 2021).
1295:
999:The drug is usually applied together with
705:
682:
571:
1361:
1251:
1200:Learn how and when to remove this message
1118:to eptifibatide and/or other ingredients.
1076:Learn how and when to remove this message
971:Learn how and when to remove this message
591:
127:Learn how and when to remove this message
1301:
678:
551:
203:
14:
2405:
1384:
696:
367:
342:
221:
1452:
651:
631:
355:
255:
238:
189:
1444:. U.S. National Library of Medicine.
1421:"From Bites and Stings to Medicines"
1178:adding citations to reliable sources
1145:
1054:adding citations to reliable sources
1021:
949:adding citations to reliable sources
916:
469:-cysteinamide, cyclic (1→6)disulfide
302:
65:adding citations to reliable sources
36:
611:
502:
24:
2433:Drugs developed by Merck & Co.
2428:Drugs developed by Schering-Plough
994:percutaneous coronary intervention
893:). It belongs to the class of the
25:
2454:
1401:
1018:Contraindications and precautions
329:
2413:Glycoprotein IIb/IIIa inhibitors
2390:
2086:Direct thrombin (IIa) inhibitors
1514:Glycoprotein IIb/IIIa inhibitors
1283:Glycoprotein IIb/IIIa inhibitors
1242:
1150:
1026:
921:
736:
730:
156:
147:
41:
1709:Thromboxane synthase inhibitors
1385:Allday, Erin (August 1, 2006).
1302:Gribble GW (15 December 2010).
1141:
1106:) is an alternative medication.
868:glycoprotein IIb/IIIa inhibitor
829:Key:CZKPOZZJODAYPZ-LROMGURASA-N
52:needs additional citations for
1425:The Royal Society of Chemistry
1378:
1329:
912:
748:
742:
724:
13:
1:
1288:
1947:Low-molecular-weight heparin
1758:Phosphodiesterase inhibitors
1671:Acetylsalicylic acid/Aspirin
1260:
890:Sistrurus miliarius barbouri
7:
1276:
909:entered the global market.
10:
2459:
1308:. Springer. pp. 11–.
1271:Millennium Pharmaceuticals
860:Millennium Pharmaceuticals
714:Chemical and physical data
29:
2341:
2313:
2214:
2157:
2084:
2036:
1925:
1919:(with some II inhibition)
1913:
1823:
1814:
1784:
1756:
1699:
1661:
1623:
1555:
1512:
1503:
1409:"Eptifibatid/Intregrilin"
870:class. Eptifibatide is a
837:
812:
792:
770:
757:
718:
713:
694:
662:
642:
622:
602:
582:
562:
542:
522:
497:
477:
437:
432:
420:
407:
397:
387:
379:
319:
314:
289:
275:
211:
197:
179:
169:
164:
155:
146:
30:Not to be confused with
1442:Drug Information Portal
1413:Pharmazeutische Zeitung
895:arginin-glycin-aspartat
2229:Plasminogen activators
1701:Thromboxane inhibitors
1625:Prostaglandin analogue
1342:The Permanente Journal
1252:Additional information
1096:Chronic kidney disease
2291:serine endopeptidases
1825:Vitamin K antagonists
1121:Severe, uncontrolled
985:myocardial infarction
2038:Direct Xa inhibitors
1870:Ethyl biscoumacetate
1736:Receptor antagonists
1174:improve this section
1050:improve this section
945:improve this section
905:and the non-peptide
61:improve this article
1681:Carbasalate calcium
987:) in patients with
351:(Prescription only)
338:(Prescription only)
143:
2368:Never to phase III
2216:Thrombolytic drugs
1932:glycosaminoglycans
1505:Antiplatelet drugs
1492:antiplatelet drugs
1354:10.7812/TPP/21.125
139:
2378:
2377:
2210:
2209:
2080:
2079:
1810:
1809:
1431:on 27 April 2006.
1315:978-3-642-15732-5
1265:Eptifibatide was
1210:
1209:
1202:
1086:
1085:
1078:
981:
980:
973:
864:antiplatelet drug
849:
848:
783:Interactive image
664:CompTox Dashboard
468:
464:
460:
456:
452:
371:
359:
346:
333:
258:
241:
224:
137:
136:
129:
111:
27:Antiplatelet drug
16:(Redirected from
2450:
2395:
2394:
2393:
2386:
2190:Drotrecogin alfa
2170:Antithrombin III
2021:Dermatan sulfate
1994:Oligosaccharides
1923:
1922:
1821:
1820:
1557:ADP receptor/P2Y
1510:
1509:
1473:
1466:
1459:
1450:
1449:
1445:
1432:
1427:. Archived from
1416:
1395:
1394:
1382:
1376:
1375:
1365:
1333:
1327:
1326:
1324:
1322:
1299:
1205:
1198:
1194:
1191:
1185:
1154:
1146:
1090:Thrombocytopenia
1081:
1074:
1070:
1067:
1061:
1030:
1022:
976:
969:
965:
962:
956:
925:
917:
886:
845:
844:
785:
765:
750:
744:
738:
732:
726:
709:
698:
687:
686:
672:
670:
655:
635:
615:
595:
575:
555:
535:
515:
505:
504:
490:
466:
462:
458:
454:
450:
412:
369:
366:
357:
354:
344:
341:
331:
328:
306:
268:
257:
254:
251:
240:
237:
234:
223:
220:
207:
193:
160:
151:
144:
142:
138:
132:
125:
121:
118:
112:
110:
69:
45:
37:
21:
2458:
2457:
2453:
2452:
2451:
2449:
2448:
2447:
2418:Cyclic peptides
2403:
2402:
2401:
2391:
2389:
2381:
2379:
2374:
2373:
2358:Clinical trials
2337:
2309:
2219:
2206:
2153:
2076:
2032:
1935:
1930:
1918:
1909:
1885:1,3-Indandiones
1827:
1806:
1780:
1752:
1695:
1657:
1619:
1571:Thienopyridines
1560:
1551:
1499:
1480:Antithrombotics
1477:
1436:
1419:
1407:
1404:
1399:
1398:
1383:
1379:
1334:
1330:
1320:
1318:
1316:
1300:
1296:
1291:
1279:
1263:
1254:
1245:
1206:
1195:
1189:
1186:
1171:
1155:
1144:
1082:
1071:
1065:
1062:
1047:
1031:
1020:
989:unstable angina
977:
966:
960:
957:
942:
926:
915:
882:
877:derived from a
840:
838:
833:
830:
825:
820:
819:
808:
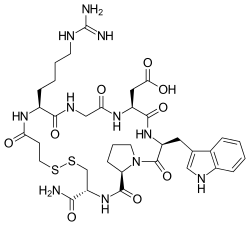
805:
800:
799:
788:
763:
753:
747:
741:
735:
729:
690:
666:
658:
638:
618:
598:
578:
558:
538:
518:
501:
493:
473:
470:
445:
444:
410:
399:Protein binding
389:Bioavailability
381:Pharmacokinetic
375:
310:
278:
271:
133:
122:
116:
113:
70:
68:
58:
46:
35:
28:
23:
22:
15:
12:
11:
5:
2456:
2446:
2445:
2440:
2435:
2430:
2425:
2420:
2415:
2400:
2399:
2376:
2375:
2372:
2371:
2370:
2369:
2366:
2355:
2349:
2343:
2342:
2339:
2338:
2336:
2335:
2330:
2325:
2319:
2317:
2311:
2310:
2308:
2307:
2302:
2297:
2287:
2282:
2277:
2272:
2271:
2270:
2265:
2257:
2256:
2255:
2250:
2245:
2240:
2225:
2223:
2212:
2211:
2208:
2207:
2205:
2204:
2199:
2194:
2193:
2192:
2182:
2177:
2172:
2167:
2161:
2159:
2155:
2154:
2152:
2151:
2146:
2141:
2136:
2131:
2126:
2117:
2116:
2115:
2110:
2105:
2090:
2088:
2082:
2081:
2078:
2077:
2075:
2074:
2069:
2064:
2059:
2054:
2049:
2043:
2041:
2034:
2033:
2031:
2030:
2029:
2028:
2023:
2018:
2008:
2007:
2006:
2001:
1991:
1990:
1989:
1984:
1979:
1974:
1969:
1964:
1959:
1954:
1943:
1941:
1920:
1911:
1910:
1908:
1907:
1901:
1896:
1891:
1882:
1877:
1872:
1867:
1862:
1857:
1847:
1845:
1818:
1816:Anticoagulants
1812:
1811:
1808:
1807:
1805:
1804:
1799:
1794:
1788:
1786:
1782:
1781:
1779:
1778:
1773:
1768:
1762:
1760:
1754:
1753:
1751:
1750:
1749:
1748:
1743:
1733:
1732:
1731:
1726:
1721:
1705:
1703:
1697:
1696:
1694:
1693:
1688:
1683:
1678:
1673:
1667:
1665:
1663:COX inhibitors
1659:
1658:
1656:
1655:
1650:
1645:
1640:
1634:
1632:
1621:
1620:
1618:
1617:
1616:
1615:
1610:
1605:
1590:
1589:
1588:
1583:
1578:
1567:
1565:
1558:
1553:
1552:
1550:
1549:
1544:
1539:
1534:
1529:
1524:
1518:
1516:
1507:
1501:
1500:
1488:anticoagulants
1476:
1475:
1468:
1461:
1453:
1447:
1446:
1438:"Eptifibatide"
1434:
1417:
1403:
1402:External links
1400:
1397:
1396:
1377:
1328:
1314:
1293:
1292:
1290:
1287:
1286:
1285:
1278:
1275:
1262:
1259:
1253:
1250:
1244:
1241:
1234:Additionally,
1208:
1207:
1158:
1156:
1149:
1143:
1140:
1139:
1138:
1135:
1132:
1129:
1126:
1119:
1113:
1110:
1107:
1093:
1084:
1083:
1034:
1032:
1025:
1019:
1016:
979:
978:
961:September 2020
929:
927:
920:
914:
911:
847:
846:
835:
834:
832:
831:
828:
826:
823:
815:
814:
813:
810:
809:
807:
806:
803:
795:
794:
793:
790:
789:
787:
786:
778:
776:
768:
767:
761:
755:
754:
751:
745:
739:
733:
727:
722:
716:
715:
711:
710:
700:
692:
691:
689:
688:
675:
673:
660:
659:
657:
656:
648:
646:
640:
639:
637:
636:
628:
626:
620:
619:
617:
616:
608:
606:
600:
599:
597:
596:
588:
586:
580:
579:
577:
576:
568:
566:
560:
559:
557:
556:
548:
546:
540:
539:
537:
536:
528:
526:
520:
519:
517:
516:
508:
506:
495:
494:
492:
491:
483:
481:
475:
474:
472:
471:
448:
440:
439:
438:
435:
434:
430:
429:
424:
418:
417:
414:
405:
404:
401:
395:
394:
391:
385:
384:
377:
376:
374:
373:
364:
352:
339:
325:
323:
317:
316:
312:
311:
309:
308:
295:
293:
287:
286:
281:
279:administration
273:
272:
270:
269:
252:
235:
217:
215:
209:
208:
201:
195:
194:
187:
177:
176:
173:
167:
166:
162:
161:
153:
152:
135:
134:
76:"Eptifibatide"
49:
47:
40:
26:
9:
6:
4:
3:
2:
2455:
2444:
2441:
2439:
2436:
2434:
2431:
2429:
2426:
2424:
2421:
2419:
2416:
2414:
2411:
2410:
2408:
2398:
2388:
2387:
2384:
2367:
2365:
2362:
2361:
2359:
2356:
2353:
2350:
2348:
2345:
2344:
2340:
2334:
2331:
2329:
2326:
2324:
2321:
2320:
2318:
2316:
2315:Non-medicinal
2312:
2306:
2303:
2301:
2298:
2296:
2292:
2288:
2286:
2285:Streptokinase
2283:
2281:
2278:
2276:
2273:
2269:
2266:
2264:
2261:
2260:
2258:
2254:
2251:
2249:
2246:
2244:
2241:
2239:
2236:
2235:
2234:
2230:
2227:
2226:
2224:
2222:
2221:fibrinolytics
2217:
2213:
2203:
2200:
2198:
2195:
2191:
2188:
2187:
2186:
2183:
2181:
2178:
2176:
2173:
2171:
2168:
2166:
2163:
2162:
2160:
2156:
2150:
2147:
2145:
2142:
2140:
2137:
2135:
2132:
2130:
2127:
2125:
2121:
2118:
2114:
2111:
2109:
2106:
2104:
2101:
2100:
2099:
2095:
2092:
2091:
2089:
2087:
2083:
2073:
2070:
2068:
2065:
2063:
2060:
2058:
2055:
2053:
2050:
2048:
2045:
2044:
2042:
2039:
2035:
2027:
2024:
2022:
2019:
2017:
2014:
2013:
2012:
2009:
2005:
2002:
2000:
1997:
1996:
1995:
1992:
1988:
1985:
1983:
1980:
1978:
1975:
1973:
1970:
1968:
1965:
1963:
1960:
1958:
1955:
1953:
1950:
1949:
1948:
1945:
1944:
1942:
1939:
1933:
1928:
1924:
1921:
1916:
1912:
1906:
1902:
1900:
1897:
1895:
1892:
1890:
1886:
1883:
1881:
1878:
1876:
1875:Phenprocoumon
1873:
1871:
1868:
1866:
1863:
1861:
1860:Coumatetralyl
1858:
1856:
1855:Acenocoumarol
1852:
1849:
1848:
1846:
1843:
1839:
1835:
1831:
1826:
1822:
1819:
1817:
1813:
1803:
1800:
1798:
1795:
1793:
1790:
1789:
1787:
1783:
1777:
1774:
1772:
1769:
1767:
1764:
1763:
1761:
1759:
1755:
1747:
1744:
1742:
1739:
1738:
1737:
1734:
1730:
1727:
1725:
1722:
1719:
1715:
1712:
1711:
1710:
1707:
1706:
1704:
1702:
1698:
1692:
1689:
1687:
1684:
1682:
1679:
1677:
1674:
1672:
1669:
1668:
1666:
1664:
1660:
1654:
1651:
1649:
1646:
1644:
1641:
1639:
1636:
1635:
1633:
1630:
1626:
1622:
1614:
1611:
1609:
1606:
1604:
1601:
1600:
1598:
1594:
1591:
1587:
1584:
1582:
1579:
1577:
1574:
1573:
1572:
1569:
1568:
1566:
1564:
1561:
1554:
1548:
1545:
1543:
1540:
1538:
1535:
1533:
1530:
1528:
1525:
1523:
1520:
1519:
1517:
1515:
1511:
1508:
1506:
1502:
1497:
1493:
1489:
1485:
1484:thrombolytics
1481:
1474:
1469:
1467:
1462:
1460:
1455:
1454:
1451:
1443:
1439:
1435:
1430:
1426:
1422:
1418:
1414:
1410:
1406:
1405:
1392:
1388:
1381:
1373:
1369:
1364:
1359:
1355:
1351:
1347:
1343:
1339:
1332:
1317:
1311:
1307:
1306:
1298:
1294:
1284:
1281:
1280:
1274:
1272:
1268:
1258:
1249:
1243:Study results
1240:
1237:
1232:
1230:
1226:
1222:
1217:
1215:
1204:
1201:
1193:
1190:November 2016
1183:
1179:
1175:
1169:
1168:
1164:
1159:This section
1157:
1153:
1148:
1147:
1136:
1133:
1130:
1127:
1124:
1120:
1117:
1114:
1111:
1108:
1105:
1102:(trade name:
1101:
1097:
1094:
1091:
1088:
1087:
1080:
1077:
1069:
1066:November 2016
1059:
1055:
1051:
1045:
1044:
1040:
1035:This section
1033:
1029:
1024:
1023:
1015:
1012:
1010:
1006:
1002:
997:
995:
990:
986:
975:
972:
964:
954:
950:
946:
940:
939:
935:
930:This section
928:
924:
919:
918:
910:
908:
904:
900:
896:
892:
891:
885:
880:
876:
873:
869:
865:
861:
857:
853:
843:
836:
827:
822:
821:
818:
811:
802:
801:
798:
791:
784:
780:
779:
777:
774:
769:
762:
760:
756:
723:
721:
717:
712:
708:
704:
701:
699:
697:ECHA InfoCard
693:
685:
681:
680:DTXSID7046673
677:
676:
674:
665:
661:
654:
650:
649:
647:
645:
641:
634:
630:
629:
627:
625:
621:
614:
610:
609:
607:
605:
601:
594:
590:
589:
587:
585:
581:
574:
570:
569:
567:
565:
561:
554:
550:
549:
547:
545:
541:
534:
530:
529:
527:
525:
521:
514:
510:
509:
507:
500:
496:
489:
485:
484:
482:
480:
476:
453:-lysylglycyl-
447:
446:
443:
436:
431:
428:
425:
423:
419:
415:
413:
406:
402:
400:
396:
392:
390:
386:
382:
378:
372: Rx-only
365:
363:
353:
350:
340:
337:
327:
326:
324:
322:
318:
313:
305:
300:
297:
296:
294:
292:
288:
285:
282:
280:
274:
267:
263:
253:
250:
245:
236:
233:
228:
219:
218:
216:
214:
210:
206:
202:
200:
196:
192:
188:
186:
182:
178:
174:
172:
168:
165:Clinical data
163:
159:
154:
150:
145:
131:
128:
120:
117:November 2016
109:
106:
102:
99:
95:
92:
88:
85:
81:
78: –
77:
73:
72:Find sources:
66:
62:
56:
55:
50:This article
48:
44:
39:
38:
33:
19:
2305:Fibrinolysin
2275:Anistreplase
2253:Desmoteplase
2248:Tenecteplase
2149:Ximelagatran
1999:Fondaparinux
1938:antithrombin
1894:Diphenadione
1771:Dipyridamole
1714:Dipyridamole
1653:Treprostinil
1648:Prostacyclin
1527:Eptifibatide
1526:
1441:
1429:the original
1424:
1415:(in German).
1412:
1390:
1380:
1345:
1341:
1331:
1319:. Retrieved
1304:
1297:
1264:
1255:
1246:
1233:
1218:
1214:side effects
1211:
1196:
1187:
1172:Please help
1160:
1142:Side effects
1123:hypertension
1072:
1063:
1048:Please help
1036:
1013:
998:
982:
967:
958:
943:Please help
931:
888:
875:heptapeptide
855:
852:Eptifibatide
851:
850:
839:
633:CHEBI:291902
461:-tryptophyl-
457:-α-aspartyl-
409:Elimination
321:Legal status
315:Legal status
266:Eptifibatide
249:Eptifibatide
213:License data
141:Eptifibatide
123:
114:
104:
97:
90:
83:
71:
59:Please help
54:verification
51:
2423:Tryptamines
2354:from market
2175:Defibrotide
2103:Bivalirudin
2072:Rivaroxaban
2011:Heparinoids
2004:Idraparinux
1905:Tioclomarol
1899:Phenindione
1889:Clorindione
1792:Cloricromen
1586:Ticlopidine
1576:Clopidogrel
1321:12 November
1236:hypotension
1225:transfusion
1005:clopidogrel
913:Indications
879:disintegrin
766: g·mol
703:100.169.160
488:188627-80-7
433:Identifiers
284:Intravenous
199:MedlinePlus
171:Trade names
32:Epibatidine
2443:Guanidines
2407:Categories
2280:Monteplase
2197:Ramatroban
2180:Nafamostat
2165:Abelacimab
2144:Melagatran
2129:Dabigatran
2124:Argatroban
2067:Otamixaban
2052:Betrixaban
2040:("xabans")
2026:Sulodexide
2016:Danaparoid
1987:Tinzaparin
1977:Parnaparin
1972:Nadroparin
1967:Enoxaparin
1962:Dalteparin
1957:Certoparin
1917:inhibitors
1865:Dicoumarol
1766:Cilostazol
1746:Terutroban
1724:Picotamide
1613:Ticagrelor
1597:nucleoside
1593:Nucleotide
1563:inhibitors
1542:Sibrafiban
1391:sfgate.com
1348:(4): 1–3.
1289:References
1267:discovered
856:Integrilin
771:3D model (
759:Molar mass
653:ChEMBL1174
593:NA8320J834
564:ChemSpider
524:IUPHAR/BPS
479:CAS Number
442:IUPAC name
416:~2.5 hours
175:Integrilin
87:newspapers
18:Integrilin
2364:Phase III
2352:Withdrawn
2268:Urokinase
2263:Saruplase
2243:Reteplase
2238:Alteplase
2185:Protein C
2139:Inogatran
2134:Efegatran
2120:Univalent
2113:Lepirudin
2108:Desirudin
2057:Darexaban
1982:Reviparin
1952:Bemiparin
1915:Factor Xa
1851:Coumarins
1828:(inhibit
1802:Vorapaxar
1776:Triflusal
1741:Terbogrel
1729:Terbogrel
1718:+ aspirin
1691:Triflusal
1686:Indobufen
1676:Aloxiprin
1638:Beraprost
1608:Elinogrel
1603:Cangrelor
1581:Prasugrel
1547:Tirofiban
1537:Roxifiban
1532:Orbofiban
1522:Abciximab
1273:in 2001.
1261:Inventors
1161:does not
1100:Abciximab
1037:does not
932:does not
907:tirofiban
903:abciximab
899:half-life
881:protein (
422:Excretion
411:half-life
277:Routes of
191:Monograph
185:Drugs.com
2397:Medicine
2094:Bivalent
2062:Edoxaban
2047:Apixaban
1880:Warfarin
1797:Ditazole
1643:Iloprost
1599:analogs
1372:35348110
1277:See also
1221:bleeding
842:(verify)
573:10482060
544:DrugBank
465:-prolyl-
291:ATC code
244:DailyMed
2333:Oxalate
2323:Citrate
2300:Brinase
2098:Hirudin
1927:Heparin
1903:Other:
1363:8784086
1182:removed
1167:sources
1116:Allergy
1058:removed
1043:sources
1009:heparin
1001:aspirin
996:(PCI).
953:removed
938:sources
866:of the
720:Formula
553:DB00063
499:PubChem
307:)
301: (
299:B01AC16
264::
246::
229::
205:a601210
101:scholar
2383:Portal
2347:WHO-EM
2295:Ancrod
2289:Other
1936:(bind
1929:group/
1370:
1360:
1312:
1229:anemia
1104:Reopro
884:P22827
872:cyclic
797:SMILES
764:831.97
644:ChEMBL
613:D06888
513:123610
427:Kidney
362:℞-only
360:
347:
334:
259:
242:
232:by INN
225:
103:
96:
89:
82:
74:
2233:r-tPA
2158:Other
1785:Other
817:InChI
773:JSmol
624:ChEBI
108:JSTOR
94:books
2328:EDTA
2259:UPA
2202:REG1
1629:PGI2
1490:and
1368:PMID
1323:2010
1310:ISBN
1165:any
1163:cite
1041:any
1039:cite
936:any
934:cite
604:KEGG
584:UNII
533:6585
403:~25%
383:data
181:AHFS
80:news
1834:VII
1496:B01
1494:) (
1358:PMC
1350:doi
1176:by
1052:by
1003:or
947:by
669:EPA
503:CID
393:n/a
349:POM
304:WHO
262:FDA
227:EMA
63:by
2409::
2360::
2293::
2231::
2122::
2096::
1887::
1853::
1840:,
1838:IX
1836:,
1832:,
1830:II
1559:12
1486:,
1440:.
1423:.
1411:.
1389:.
1366:.
1356:.
1346:25
1344:.
1340:.
858:,
740:11
734:49
728:35
368:EU
356:US
343:UK
336:S4
330:AU
256:US
239:US
222:EU
2385::
2218:/
1940:)
1934:/
1844:)
1842:X
1720:)
1716:(
1631:)
1627:(
1595:/
1498:)
1482:(
1472:e
1465:t
1458:v
1393:.
1374:.
1352::
1325:.
1203:)
1197:(
1192:)
1188:(
1184:.
1170:.
1125:.
1079:)
1073:(
1068:)
1064:(
1060:.
1046:.
974:)
968:(
963:)
959:(
955:.
941:.
854:(
775:)
752:2
749:S
746:9
743:O
737:N
731:H
725:C
671:)
667:(
467:L
463:L
459:L
455:L
451:L
370::
358::
345::
332::
183:/
130:)
124:(
119:)
115:(
105:·
98:·
91:·
84:·
57:.
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.