691:
712:
624:
30:
332:
22:
619:{\displaystyle {\begin{aligned}{\ce {MCl}}_{n}+n\,{\ce {HOTf}}&\longrightarrow {\ce {M(OTf)}}_{n}+n\,{\ce {HCl}}\\{\ce {MCl}}_{n}+n\,{\ce {AgOTf}}&\longrightarrow {\ce {M(OTf)}}_{n}+n\,{\ce {AgCl \, v}}\\{\ce {M(SO4)}}_{n}+n\,{\ce {Ba(OTf)2}}&\longrightarrow {\ce {M(OTf)}}_{2n}+n\,{\ce {BaSO4 v}}\end{aligned}}}
318:
salts especially in water-free form. They can be obtained directly from triflic acid and the metal hydroxide or metal carbonate in water. Alternatively, they can be obtained from reacting metal chlorides with neat triflic acid or
847:
Aihara, Yoshinori; Chatani, Naoto (10 April 2013). "Nickel-Catalyzed Direct
Alkylation of C–H Bonds in Benzamides and Acrylamides with Functionalized Alkyl Halides via Bidentate-Chelation Assistance".
337:
324:
690:
833:
777:
Dixon, N. E.; Lawrance, G. A.; Lay, P. A.; Sargeson, A. M.; Taube, H. (1990). "Trifluoromethanesulfonates and
Trifluoromethanesulfonato-
239:
which causes the negative charge to be spread symmetrically over the three oxygen atoms. An additional stabilization is achieved by the
284:
913:
804:
742:
244:
705:
208:
893:
664:
660:
898:
888:
236:
95:
269:
637:
49:
8:
783:
903:
752:
747:
75:
864:
800:
652:
633:
204:
41:
776:
856:
829:
792:
757:
672:
303:
265:
255:
251:
61:
57:
908:
732:
320:
240:
212:
796:
680:
656:
160:
882:
676:
216:
200:
184:
868:
834:
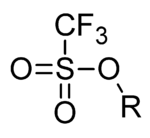
10.1002/(SICI)1099-0690(199901)1999:1<15::AID-EJOC15>3.0.CO;2-B
668:
220:
164:
273:
228:
110:
648:
629:
259:
860:
737:
180:
306:
are thermally very stable with melting points up to 350 °C for
711:
684:
29:
820:
Kobayashi, S. (1999). "Scandium
Triflate in Organic Synthesis".
315:
307:
311:
232:
34:
188:
21:
603:
548:
505:
140:
250:
Triflates have also been applied as ligands for group
335:
618:
219:. Since alkyl triflates are extremely reactive in
880:
16:Chemical group (–OSO2CF3) or anion (charge –1)
846:
819:
88:. The triflate group is often represented by
227:, they must be stored in conditions free of
591:
525:
475:
470:
428:
400:
358:
849:Journal of the American Chemical Society
710:
28:
20:
881:
683:. The corresponding reaction with the
822:European Journal of Organic Chemistry
292:-bis(trifluoromethanesulfonyl)aniline
722:to facilitate C–H functionalization
247:using the sulfur atom as a bridge.
235:). The anion owes its stability to
183:; i.e. it is more acidic than pure
13:
689:
94:, as opposed to −Tf, which is the
14:
925:
297:
199:A triflate group is an excellent
163:; this comes from the fact that
194:
187:, already one of the strongest
840:
813:
770:
651:). A related popular catalyst
607:
571:
565:
556:
538:
532:
508:
492:
477:
453:
447:
438:
383:
377:
368:
327:with metal sulfates in water:
243:group, which acts as a strong
1:
763:
743:Trifluoromethanesulfonic acid
655:is used in such reactions as
704:Triflate is a commonly used
700:-mediated aldol condensation
636:. Especially useful are the
628:Metal triflates are used as
294:, where the by-product is .
7:
726:
139:The corresponding triflate
116:triflate can be written as
10:
930:
797:10.1002/9780470132593.ch16
245:electron-withdrawing group
914:Trifluoromethyl compounds
706:weakly coordinating anion
209:nucleophilic substitution
159:, is an extremely stable
54:trifluoromethanesulfonate
665:Mukaiyama aldol addition
283:is phenyl triflimide or
237:resonance stabilization
723:
701:
620:
272:as a component of the
37:
26:
714:
693:
661:Diels–Alder reactions
621:
270:lithium ion batteries
32:
24:
663:. An example is the
638:lanthanide triflates
333:
784:Inorganic Syntheses
605:
550:
507:
323:, or from reacting
748:Metal triflimidate
724:
702:
616:
614:
593:
530:
495:
281:triflating reagent
258:metals along with
38:
27:
861:10.1021/ja401344e
855:(14): 5308–5311.
667:reaction between
653:scandium triflate
634:organic chemistry
596:
570:
563:
537:
529:
498:
490:
474:
452:
445:
432:
414:
404:
382:
375:
362:
344:
268:are used in some
266:Lithium triflates
205:organic reactions
42:organic chemistry
921:
873:
872:
844:
838:
837:
817:
811:
810:
774:
758:Lithium triflate
721:
699:
673:silyl enol ether
646:
625:
623:
622:
617:
615:
611:
610:
604:
601:
594:
584:
583:
575:
574:
568:
561:
551:
549:
546:
541:
535:
527:
518:
517:
512:
511:
506:
503:
496:
488:
481:
480:
472:
463:
462:
457:
456:
450:
443:
433:
430:
421:
420:
415:
412:
405:
402:
393:
392:
387:
386:
380:
373:
363:
360:
351:
350:
345:
342:
213:Suzuki couplings
203:used in certain
178:
158:
157:
156:
153:
135:
108:
92:
87:
73:
58:functional group
929:
928:
924:
923:
922:
920:
919:
918:
894:Sulfonyl groups
879:
878:
877:
876:
845:
841:
818:
814:
807:
775:
771:
766:
753:Comins' reagent
733:Methyl triflate
729:
720:
716:
698:
694:
657:aldol reactions
647:(where Ln is a
645:
641:
613:
612:
606:
602:
597:
592:
576:
564:
560:
559:
552:
547:
542:
531:
526:
513:
504:
499:
491:
487:
486:
483:
482:
476:
471:
458:
446:
442:
441:
434:
429:
416:
411:
410:
407:
406:
401:
388:
376:
372:
371:
364:
359:
346:
341:
340:
336:
334:
331:
330:
325:barium triflate
321:silver triflate
300:
241:trifluoromethyl
224:
197:
176:
172:
168:
154:
151:
150:
148:
144:
133:
129:
125:
121:
117:
109:. For example,
107:
103:
99:
90:
86:
82:
78:
72:
68:
64:
50:systematic name
17:
12:
11:
5:
927:
917:
916:
911:
906:
901:
899:Leaving groups
896:
891:
889:Sulfonic acids
875:
874:
839:
812:
805:
768:
767:
765:
762:
761:
760:
755:
750:
745:
740:
735:
728:
725:
718:
696:
681:chemical yield
643:
609:
600:
590:
587:
582:
579:
573:
567:
558:
555:
553:
545:
540:
534:
524:
521:
516:
510:
502:
494:
485:
484:
479:
469:
466:
461:
455:
449:
440:
437:
435:
427:
424:
419:
409:
408:
399:
396:
391:
385:
379:
370:
367:
365:
357:
354:
349:
339:
338:
299:
298:Triflate salts
296:
222:
217:Heck reactions
196:
193:
174:
170:
161:polyatomic ion
146:
131:
127:
123:
119:
105:
101:
84:
80:
70:
66:
25:Triflate group
15:
9:
6:
4:
3:
2:
926:
915:
912:
910:
907:
905:
902:
900:
897:
895:
892:
890:
887:
886:
884:
870:
866:
862:
858:
854:
850:
843:
835:
831:
827:
823:
816:
808:
806:9780470132593
802:
798:
794:
790:
786:
785:
780:
773:
769:
759:
756:
754:
751:
749:
746:
744:
741:
739:
736:
734:
731:
730:
713:
709:
707:
692:
688:
686:
682:
678:
677:cyclohexanone
674:
670:
666:
662:
658:
654:
650:
639:
635:
632:catalysts in
631:
626:
598:
588:
585:
580:
577:
554:
543:
522:
519:
514:
500:
467:
464:
459:
436:
425:
422:
417:
397:
394:
389:
366:
355:
352:
347:
328:
326:
322:
317:
313:
309:
305:
295:
293:
291:
287:
282:
277:
275:
271:
267:
263:
261:
257:
253:
248:
246:
242:
238:
234:
230:
226:
218:
214:
210:
206:
202:
201:leaving group
192:
190:
186:
185:sulfuric acid
182:
166:
162:
142:
137:
115:
113:
97:
96:triflyl group
93:
77:
63:
59:
55:
51:
47:
43:
36:
31:
23:
19:
852:
848:
842:
828:(1): 15–27.
825:
821:
815:
788:
782:
781:Complexes".
778:
772:
703:
687:salt fails:
679:with an 81%
669:benzaldehyde
640:of the type
627:
329:
301:
289:
285:
280:
278:
264:
249:
229:nucleophiles
198:
195:Applications
165:triflic acid
138:
111:
89:
53:
45:
39:
18:
274:electrolyte
260:lanthanides
225:2 reactions
883:Categories
764:References
649:lanthanoid
630:Lewis acid
904:Triflates
791:: 70–76.
738:Nonaflate
608:↓
557:⟶
478:↓
439:⟶
369:⟶
302:Triflate
231:(such as
181:superacid
79:R−O−S(=O)
76:structure
60:with the
33:Triflate
869:23495861
727:See also
671:and the
207:such as
56:), is a
46:triflate
717:Ni(OTf)
715:Use of
695:Sc(OTf)
685:yttrium
642:Ln(OTf)
279:A mild
191:known.
179:) is a
62:formula
909:Anions
867:
803:
316:silver
308:sodium
114:-butyl
431:AgOTf
312:boron
304:salts
233:water
189:acids
141:anion
65:R−OSO
35:anion
865:PMID
826:1999
801:ISBN
659:and
595:BaSO
473:AgCl
361:HOTf
314:and
254:and
215:and
100:R−SO
91:−OTf
74:and
857:doi
853:135
830:doi
793:doi
675:of
569:OTf
536:OTf
451:OTf
413:MCl
403:HCl
381:OTf
343:MCl
134:OTf
83:−CF
40:In
885::
863:.
851:.
824:.
799:.
789:28
787:.
708:.
528:Ba
497:SO
310:,
276:.
262:.
256:13
252:11
211:,
173:SO
169:CF
149:SO
145:CF
143:,
136:.
130:CH
126:CH
122:CH
118:CH
104:CF
98:,
69:CF
52::
44:,
871:.
859::
836:.
832::
809:.
795::
779:O
719:2
697:3
644:3
599:4
589:n
586:+
581:n
578:2
572:)
566:(
562:M
544:2
539:)
533:(
523:n
520:+
515:n
509:)
501:4
493:(
489:M
468:n
465:+
460:n
454:)
448:(
444:M
426:n
423:+
418:n
398:n
395:+
390:n
384:)
378:(
374:M
356:n
353:+
348:n
290:N
288:,
286:N
223:N
221:S
177:H
175:3
171:3
167:(
155:3
152:−
147:3
132:2
128:2
124:2
120:3
112:n
106:3
102:2
85:3
81:2
71:3
67:2
48:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.