81:
20:
59:
defines a pharmacophore to be "an ensemble of steric and electronic features that is necessary to ensure the optimal supramolecular interactions with a specific biological target and to trigger (or block) its biological response". A pharmacophore model explains how structurally diverse ligands can
139:
The features need to match different chemical groups with similar properties, in order to identify novel ligands. Ligand-receptor interactions are typically "polar positive", "polar negative" or "hydrophobic". A well-defined pharmacophore model includes both hydrophobic volumes and hydrogen bond
155:– Choose a structurally diverse set of molecules that will be used for developing the pharmacophore model. As a pharmacophore model should be able to discriminate between molecules with and without bioactivity, the set of molecules should include both active and inactive compounds.
227:
models. Such tools and a related concept of "privileged structures", which are "defined as molecular frameworks which are able of providing useful ligands for more than one type of receptor or enzyme target by judicious structural modifications", aid in
181:– Transform the superimposed molecules into an abstract representation. For example, superimposed phenyl rings might be referred to more conceptually as an 'aromatic ring' pharmacophore element. Likewise, hydroxy groups could be designated as a '
337:
Madsen U, Bräuner-Osborne H, Greenwood JR, Johansen TN, Krogsgaard-Larsen P, Liljefors T, Nielsen M, Frølund B (2005). "GABA and
Glutamate receptor ligands and their therapeutic potential in CNS disorders". In Gad SC (ed.).
175:, phenyl rings or carboxylic acid groups). The set of conformations (one conformation from each active molecule) that results in the best fit is presumed to be the active conformation.
223:
can then be searched for more molecules which share the same features arranged in the same relative orientation. Pharmacophores are also used as the starting point for developing
56:
802:
245:
104:. Red and blue sticks are oxygen and nitrogen atoms that are present in both structures. The red spheres labeled H1 and H2/A3 are, respectively,
624:
681:
620:
The following computer software packages enable the user to model the pharmacophore using a variety of computational chemistry methods:
161:– Generate a set of low energy conformations that is likely to contain the bioactive conformation for each of the selected molecules.
136:. These pharmacophore points may be located on the ligand itself or may be projected points presumed to be located in the receptor.
447:
Kier LB (September 1967). "Molecular orbital calculation of preferred conformations of acetylcholine, muscarine, and muscarone".
363:
Duarte, CD; et al. (2007), "Privileged structures: a useful concept for the rational design of new lead drug candidates",
255:. However neither the alleged source nor any of his other works mention the term "pharmacophore" or make use of the concept.
203:
As the biological activities of new molecules become available, the pharmacophore model can be updated to further refine it.
199:. The model is only valid insofar as it is able to account for differences in biological activity of a range of molecules.
674:
604:
585:
483:
347:
823:
667:
248:, in a 1960s book, uses the expression "pharmacophoric moiety" that corresponds to the modern concept.
757:
400:"Pharmacophore-based models for therapeutic drugs against phosphorylated tau in Alzheimer's disease"
828:
215:, pharmacophores are used to define the essential features of one or more molecules with the same
167:– Superimpose ("fit") all combinations of the low-energy conformations of the molecules. Similar (
212:
61:
742:
274:
195:
accounting for the observed biological activities of a set of molecules that bind to a common
762:
244:, who mentions the concept in 1967 and uses the term in a publication in 1971. Nevertheless,
48:
44:
639:
8:
777:
767:
691:
216:
148:
The process for developing a pharmacophore model generally involves the following steps:
28:
424:
399:
546:
752:
600:
581:
479:
456:
429:
380:
343:
220:
196:
69:
32:
787:
782:
747:
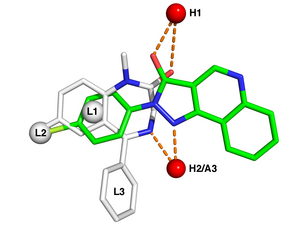
732:
527:
419:
411:
372:
317:
707:
415:
269:
264:
659:
772:
737:
376:
229:
89:
85:
817:
722:
712:
531:
182:
125:
109:
105:
52:
19:
322:
306:"Glossary of terms used in medicinal chemistry (IUPAC Recommendations 1998)"
305:
792:
644:
433:
384:
252:
460:
108:
donating and accepting sites in the receptor, while L1, L2, and L3 denote
727:
629:
241:
168:
117:
65:
64:
site. Furthermore, pharmacophore models can be used to identify through
171:) functional groups common to all molecules in the set might be fitted (
717:
336:
284:
634:
515:
121:
101:
97:
40:
649:
251:
The development of the concept is often erroneously accredited to
240:
Historically, the modern idea of pharmacophore was popularized by
96:. White sticks represent the carbon atoms of the benzodiazepine
342:. Hoboken, N.J: Wiley-Interscience/J. Wiley. pp. 797–907.
303:
129:
100:, while green represents carbon atoms of the nonbenzodiazepine
654:
578:
Pharmacophore perception, development, and use in drug design
133:
702:
279:
224:
304:
Wermuth CG, Ganellin CR, Lindberg P, Mitscher LA (1998).
80:
547:"Monty Kier and the Origin of the Pharmacophore Concept"
72:novel ligands that will bind to the same receptor.
689:
815:
467:
330:
554:Internet Electronic Journal of Molecular Design
398:Jangampalli Adi, Pradeepkiran (February 2019).
397:
297:
594:
580:. LaJolla, CA: International University Line.
675:
544:
803:Quantitative structure–activity relationship
478:. Boston: Academic Press. pp. 164–169.
356:
516:"Über den jetzigen Stand der Chemotherapie"
507:
498:
84:An example of a pharmacophore model of the
682:
668:
440:
16:Abstract description of molecular features
597:Pharmacophores and pharmacophore searches
513:
476:Molecular orbital theory in drug research
423:
321:
575:
79:
18:
473:
446:
116:Typical pharmacophore features include
816:
362:
185:donor/acceptor' pharmacophore element.
663:
492:
143:
23:An example of a pharmacophore model
13:
568:
14:
840:
615:
501:Chemobiodynamics and Drug Design
153:Select a training set of ligands
43:features that are necessary for
206:
595:Langer T, Hoffmann RD (2006).
538:
391:
39:is an abstract description of
1:
640:MOE - Pharmacophore Discovery
290:
191:– A pharmacophore model is a
416:10.1016/j.drudis.2018.11.005
7:
258:
75:
10:
845:
377:10.2174/138955707782331722
310:Pure and Applied Chemistry
235:
698:
219:. A database of diverse
165:Molecular superimposition
532:10.1002/cber.19090420105
758:Lipinski's rule of five
599:. Weinheim: WILEY-VCH.
340:Drug Discovery Handbook
323:10.1351/pac199870051129
213:computational chemistry
159:Conformational analysis
576:Güner OF, ed. (1999).
545:J.H. van Drie (2007).
275:Pharmaceutical company
113:
24:
763:Lipophilic efficiency
520:Ber. Dtsch. Chem. Ges
128:acceptors or donors,
83:
45:molecular recognition
22:
499:Schueler FW (1960).
404:Drug Discovery Today
88:binding site on the
824:Medicinal chemistry
778:New chemical entity
768:Mechanism of action
692:medicinal chemistry
217:biological activity
29:medicinal chemistry
514:Ehrlich P (1909).
221:chemical compounds
114:
25:
811:
810:
753:Ligand efficiency
371:(11): 1108–1119,
365:Mini Rev Med Chem
197:biological target
144:Model development
70:virtual screening
60:bind to a common
33:molecular biology
836:
788:Pharmacokinetics
783:Pharmacodynamics
748:Enzyme inhibitor
733:Drug development
684:
677:
670:
661:
660:
625:Discovery Studio
610:
591:
562:
561:
551:
542:
536:
535:
511:
505:
504:
496:
490:
489:
474:Kier LB (1971).
471:
465:
464:
444:
438:
437:
427:
395:
389:
388:
360:
354:
353:
334:
328:
327:
325:
316:(5): 1129–1143.
301:
51:by a biological
844:
843:
839:
838:
837:
835:
834:
833:
829:Cheminformatics
814:
813:
812:
807:
708:Bioavailability
694:
688:
618:
613:
607:
588:
571:
569:Further reading
566:
565:
549:
543:
539:
512:
508:
497:
493:
486:
472:
468:
445:
441:
396:
392:
361:
357:
350:
335:
331:
302:
298:
293:
270:Molecule mining
265:Cheminformatics
261:
238:
209:
146:
93:
78:
17:
12:
11:
5:
842:
832:
831:
826:
809:
808:
806:
805:
800:
795:
790:
785:
780:
775:
773:Mode of action
770:
765:
760:
755:
750:
745:
743:Drug targeting
740:
738:Drug discovery
735:
730:
725:
720:
715:
710:
705:
699:
696:
695:
687:
686:
679:
672:
664:
658:
657:
652:
647:
642:
637:
632:
627:
617:
616:External links
614:
612:
611:
605:
592:
586:
572:
570:
567:
564:
563:
537:
506:
503:. McGraw-Hill.
491:
484:
466:
449:Mol. Pharmacol
439:
410:(2): 616–623.
390:
355:
348:
329:
295:
294:
292:
289:
288:
287:
282:
277:
272:
267:
260:
257:
237:
234:
230:drug discovery
208:
205:
201:
200:
186:
176:
162:
156:
145:
142:
112:binding sites.
91:
86:benzodiazepine
77:
74:
66:de novo design
15:
9:
6:
4:
3:
2:
841:
830:
827:
825:
822:
821:
819:
804:
801:
799:
798:Pharmacophore
796:
794:
791:
789:
786:
784:
781:
779:
776:
774:
771:
769:
766:
764:
761:
759:
756:
754:
751:
749:
746:
744:
741:
739:
736:
734:
731:
729:
726:
724:
723:Drug delivery
721:
719:
716:
714:
713:Chemogenomics
711:
709:
706:
704:
701:
700:
697:
693:
685:
680:
678:
673:
671:
666:
665:
662:
656:
653:
651:
648:
646:
643:
641:
638:
636:
633:
631:
628:
626:
623:
622:
621:
608:
606:3-527-31250-1
602:
598:
593:
589:
587:0-9636817-6-1
583:
579:
574:
573:
559:
555:
548:
541:
533:
529:
525:
521:
517:
510:
502:
495:
487:
485:0-12-406550-3
481:
477:
470:
462:
458:
455:(5): 487–94.
454:
450:
443:
435:
431:
426:
421:
417:
413:
409:
405:
401:
394:
386:
382:
378:
374:
370:
366:
359:
351:
349:0-471-21384-5
345:
341:
333:
324:
319:
315:
311:
307:
300:
296:
286:
283:
281:
278:
276:
273:
271:
268:
266:
263:
262:
256:
254:
249:
247:
246:F. W. Shueler
243:
233:
231:
226:
222:
218:
214:
204:
198:
194:
190:
187:
184:
183:hydrogen-bond
180:
177:
174:
170:
166:
163:
160:
157:
154:
151:
150:
149:
141:
137:
135:
131:
127:
126:hydrogen bond
123:
119:
111:
107:
106:hydrogen bond
103:
99:
95:
87:
82:
73:
71:
67:
63:
58:
54:
53:macromolecule
50:
46:
42:
38:
37:pharmacophore
34:
30:
21:
797:
793:Pharmacology
619:
596:
577:
557:
553:
540:
523:
519:
509:
500:
494:
475:
469:
452:
448:
442:
407:
403:
393:
368:
364:
358:
339:
332:
313:
309:
299:
253:Paul Ehrlich
250:
239:
210:
207:Applications
202:
192:
188:
178:
172:
169:bioisosteric
164:
158:
152:
147:
138:
115:
36:
26:
728:Drug design
650:ZINCPharmer
645:ICM-Chemist
630:LigandScout
242:Lemont Kier
179:Abstraction
120:centroids,
118:hydrophobic
818:Categories
718:Drug class
690:Topics in
560:: 271–279.
291:References
211:In modern
193:hypothesis
189:Validation
140:vectors.
110:lipophilic
526:: 17–47.
285:in silico
41:molecular
434:30453058
385:18045214
259:See also
122:aromatic
102:CGS-9896
98:diazepam
94:receptor
76:Features
62:receptor
655:Pharmit
461:6052710
425:6397090
236:History
225:3D-QSAR
130:cations
124:rings,
603:
584:
482:
459:
432:
422:
383:
346:
134:anions
132:, and
49:ligand
635:Phase
550:(PDF)
57:IUPAC
47:of a
703:ADME
601:ISBN
582:ISBN
480:ISBN
457:PMID
430:PMID
381:PMID
344:ISBN
280:QSAR
173:e.g.
90:GABA
35:, a
31:and
528:doi
420:PMC
412:doi
373:doi
318:doi
68:or
27:In
820::
556:.
552:.
524:42
522:.
518:.
451:.
428:.
418:.
408:24
406:.
402:.
379:,
367:,
314:70
312:.
308:.
232:.
55:.
683:e
676:t
669:v
609:.
590:.
558:6
534:.
530::
488:.
463:.
453:3
436:.
414::
387:.
375::
369:7
352:.
326:.
320::
92:A
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.